Question
Question: Which of the following compounds does not react with \(NaHS{{O}_{3}}\)? (A)- HCHO (B)- \({{C}_{6...
Which of the following compounds does not react with NaHSO3?
(A)- HCHO
(B)- C6H5COCH3
(C)- CH3COCH3
(D)- CH3CHO
Solution
The chemical name of NaHSO3 is sodium bisulfite or sodium hydrogen sulphite. It is a white crystalline solid and has rotten egg-like smell. It reacts with compounds having carbonyl groups to form an addition-product.
Complete step by step answer:
Sodium bisulphate (or sodium hydrogen sulphite) reacts with carbonyl compounds mostly aldehydes (RCHO) and some ketones (RCOR) where the hydrocarbon chain, i.e. R-group is a small aliphatic chain like methyl ketone. Aromatic ketones generally do not react with NaHSO3 due to steric effects of large benzene ring,
NaHSO3 addition to aldehydes and ketones is a nucleophilic addition reaction. Nucleophile (HSO3−) attacks the electrophilic carbonyl carbon. A tetrahedral alkoxide is formed as an intermediate. Final proton transfer occurs to give bisulfite addition compounds. If a large or bulky R-group is present adjacent to the electrophilic carbonyl carbon then the addition of HSO3− is hindered.
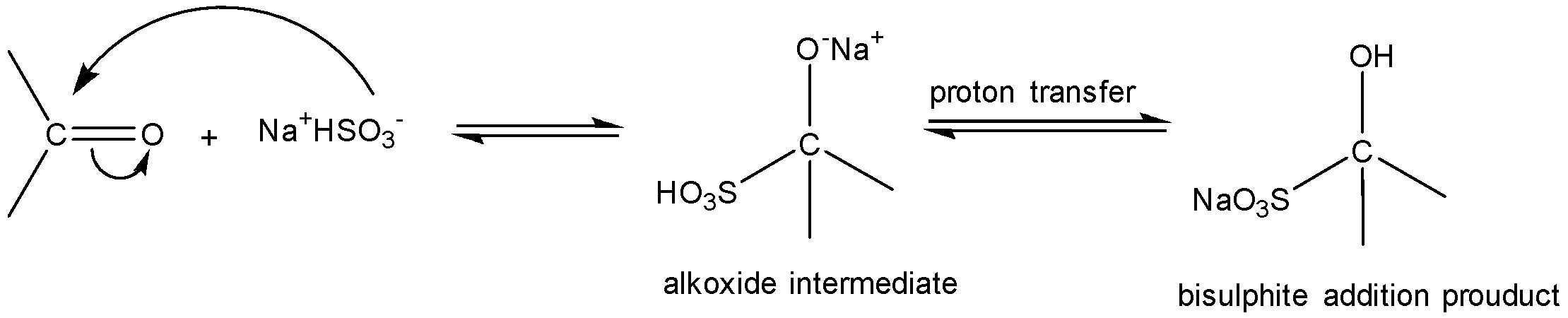
HCHO (formaldehyde), CH3CHO(acetaldehyde) are aldehydes and thus react with NaHSO3 easily to addition products. However, reactivity of ketones is less than that of aldehydes. CH3COCH3 (acetone) has only methyl substituents and HSO3− addition can occur.
C6H5COCH3 (acetophenone) has one phenyl group attached and the attack of the nucleophile is sterically favourable. Hence, C6H5COCH3 does give addition reaction with NaHSO3.
So, the correct answer is “Option B”.
Additional Information:
The addition bisulphite product formed by nucleophilic addition of NaHSO3 to carbonyl compounds is crystalline and water soluble. It is converted back to the carbonyl compound on treatment with mineral acid or base.
Note: Note that the reaction of a carbonyl compound with NaHSO3 nucleophile attack to carbonyl carbon occurs from sulphur (bisulfite ion) and not oxygen. Sulphur is less electronegative than oxygen and has lesser tendency than oxygen to hold electrons on it. Therefore, sulphur behaves as a better nucleophile than oxygen.
