Question
Question: Which of the following compounds does not give a haloform reaction? (A)  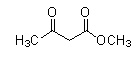
(B) 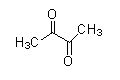
(C) 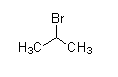
(D) 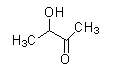
Solution
Hint: To obtain Haloform as the product, the starting material needs to have methyl ketones in order to undergo reaction in Haloform reaction. Tertiary halogen atoms do not get oxidized in conditions as given in Haloform reaction.
Complete answer:
- The compound that does not have −COCH3 cannot undergo a Haloform reaction.
As we see the structures of all compounds, we find out that only compounds given in option (C) do not have −COCH3 group. So, it cannot give this reaction.
So, Correct answer is option (C) 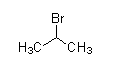
Additional Information:
- Haloform reaction converts acetyl groups to carboxylic acid functionality by exhaustive halogenations and gives various haloform as side products.
- Following is the Haloform reaction of compound given in option (A) with mechanism.
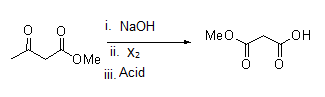
Mechanism:
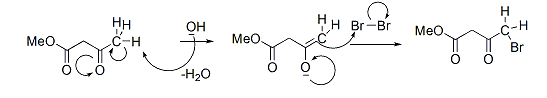
Same way, all three protons get substituted by Bromine atoms and finally Hydrolysis converts acetyl group into carboxylic acid and Haloform is produced as a side product. Here, we have used Br2 as a halogen, so respective haloforms will form.
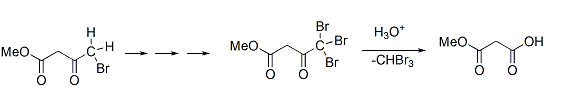
Same way compounds given in option (B) and (D) give Haloform reaction as shown under.
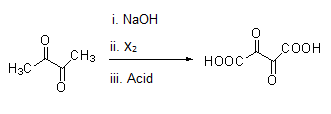
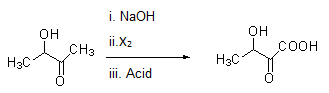
Note:
Secondary alcohol oxidizes itself in the conditions present in Haloform reaction and if it gets converted into methyl ketones, then it will give Haloform reaction even though it does not contain −COCH3 functionality. Acidic hydrolysis is necessary in order to obtain the final product.
