Question
Question: Which of the following compounds does not contain a carbonyl group? (A). Acetylacetone (B). Diet...
Which of the following compounds does not contain a carbonyl group?
(A). Acetylacetone
(B). Diethyl malonate
(C). Amines
(D). Meldrum’s acid
Solution
Organic chemistry is the branch of the chemistry that deals with the study of the hydrocarbons and several other functional groups like, halogens, alcohols, phenol, ethers, aldehydes, ketones, carboxylic acids, have nitrogen, oxygen, and other atoms as functional groups. Each functional group has a separate identity and different chemical and physical properties.
Complete answer:
Organic Chemistry has several functional groups like halogens, alcohols, phenol, ethers, aldehydes, ketones, carboxylic acids, nitrogen, oxygen, and other atoms. However, the collective group of aldehydes, ketones and carboxylic acids is known as the Carbonyl group. The common thing in all is the presence of (R)2−C=O . In case of aldehydes, one of the R is hydrogen represented as R−CH=O in case of ketones both R may be same or different having representation as (R)2−C=O or R−CR′=O in case of carboxylic acids the general representation is as R−COOH .
The acetylacetone is a carbonyl complex which has a structure as given: -
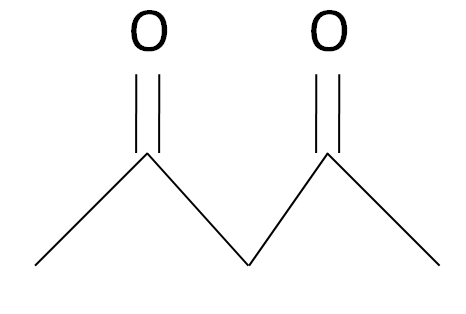
The Diethyl malonate is a carbonyl complex which has a structure as given: -
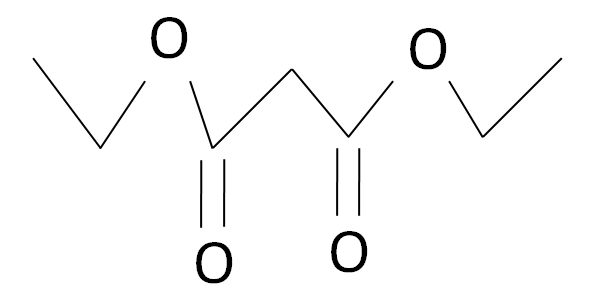
The Amine is an organic compound which does not contain any type of carbonyl group as shown: -
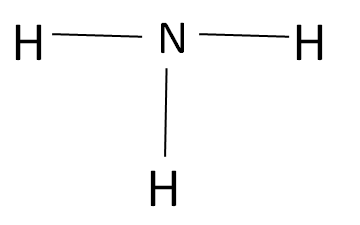
The Meldrum’s acid is a carbonyl complex which has a structured as given: -
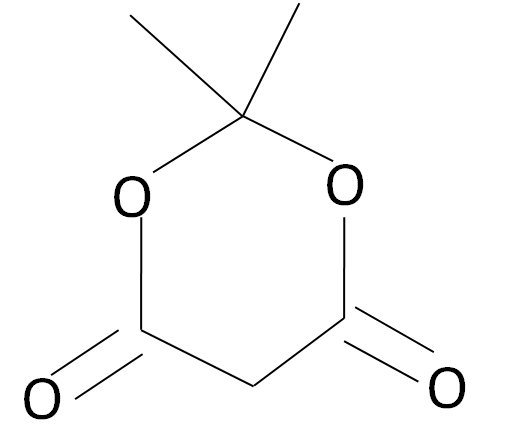
Note:
The carbonyl group is a special group in organic chemistry which consists of three different types of functional groups namely aldehydes, ketones and carboxylic acids. According to the I.U.P.A.C (International Union of Pure and Applied Chemistry) the suffixes of their names are –al for aldehydes, -One for ketones and –Oic acids for carboxylic acids.
