Question
Question: Which of the following compounds contain a single covalent bond? A. Oxygen B. Nitrogen C. Me...
Which of the following compounds contain a single covalent bond?
A. Oxygen
B. Nitrogen
C. Methane
D. Carbon dioxide
Solution
Atoms may attain a stable electronic configuration in three different ways by losing electrons, by gaining or sharing electrons. Therefore form three different types of bonds depending upon its electropositive and negative character.
Complete answer:
A covalent bond is formed between two electronegative elements by sharing the pair of electrons between its atoms. We know that electronegative elements are the elements accepting electrons, a few bonds are purely ionic, covalent or metallic. Most are intermediate between the three main types of bonding and show some properties of at least two and sometimes of all three types.
According to the question let us now discuss which of the following molecules will have a single covalent bond. Note that atoms forming covalent bonds but none of those atoms should have a tendency to lose electrons. The compounds above have a structure like,
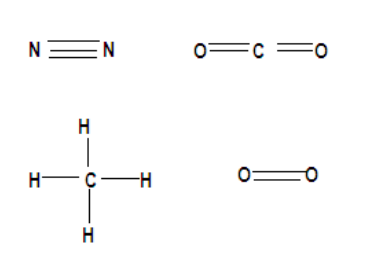
Therefore we observe that though all the compounds form covalent bonds but in the question we have been asked about single covalent bonds and all other compounds form multiple covalent bonds that are 2 in carbon dioxide, oxygen and 3 in nitrogen. It is only methane with single covalent bonds because the central carbon atom that is four electrons short of the noble gas structure, so it forms four bonds, and the hydrogen atoms are short of one electron, so they form one bond each. By sharing electrons this way both the carbon and all four hydrogen atoms attain a noble gas configuration. Therefore forming covalent bonds by accepting electrons.
**Hence the correct option is C.
Note:**
Normally only electrons in the outermost shell of an atom are involved in forming bonds, and by forming bonds each atom acquires a stable electronic configuration. The most stable electronic arrangement is a noble gas structure. However less stable arrangements in this are commonly obtained by transition elements.
