Question
Question: Which of the following compounds can form a zwitterion? [A] Benzoic acid [B] Aniline [C] Glyci...
Which of the following compounds can form a zwitterion?
[A] Benzoic acid
[B] Aniline
[C] Glycine
[D] Acetanilide
Solution
To answer this, remember that amino acids are able to form zwitterions because they contain both an amino group and a carboxyl group. In its zwitterion, the carboxyl group has a negative charge and the amine group has a positive charge thus giving rise to a dipolar ion.
Complete step by step solution:
We know that a zwitterion is a molecule which contains both positively charged and negatively charged ions in it. It is also referred to as inner salt. Zwitterion has an equal number of positively charged and negatively charged functional groups.
As zwitterions have both positive charge and negative charge thus, they are dipolar.
Generally amino acids form zwitterions in aqueous solutions. The carboxyl groups can lose a proton and gain a negative charge and the amino group can gain a proton and get a positive charge thus giving rise to a dipolar ion.
Let us discuss the structures of the compounds given and try to explain if they will form a zwitterion.
Firstly, we have benzoic acid. We know that structure of benzoic acid is-
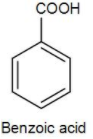
As we can see it has a carboxyl group but no amino group. The carboxyl acid can lose the proton and gain a negative charge but there will be no zwitterion formation. Therefore, it is not the correct answer.
Next, we have aniline. We can draw the structure of aniline as-
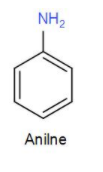
It has an amino group but no carboxyl group. Thus, it will not form a zwitterion.
Then we have glycine. The structure is-
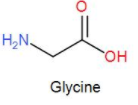
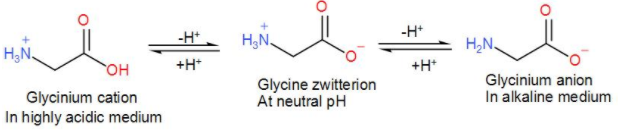
As we can see it has both, a carboxyl group as well as an amino group. Therefore, it can give rise to a dipolar ion.
And lastly, we have acetanilide. The structure is-
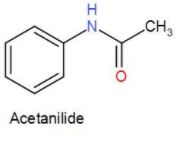
As we can see it has an amine group but no carboxylic group. Therefore, it will neither form a zwitterion.
As we can see from the above discussion that only glycine can form a zwitterion.
Therefore, the correct answer is option [C] glycine.
Note: A compound containing double functional groups, like amino acid and the carboxylic group can form a zwitterion. Therefore, generally all amino acids form zwitterion as they have both an amino group and a carboxyl group. Zwitterions can form other molecules called ampholytes which are amphoteric in nature and can act as both acid as well as base.
