Question
Question: Which of the following compounds are not soluble in water? (A)- Formaldehyde (B)- Acetaldehyde ...
Which of the following compounds are not soluble in water?
(A)- Formaldehyde
(B)- Acetaldehyde
(C)- Acetone
(D)- None of the above
Solution
The solubility of aldehydes and ketones is determined with respect to the ease with which it forms the hydrogen bonding with water to the relatively electronegative oxygen atom on the carbonyl group, making it polar.
Complete step by step answer:
The aldehydes and ketones solubility takes place through the nucleophilic addition reaction of water molecules to the carbonyl group, catalysed by either an acid or base. The oxygen being relatively electronegative atom compared to the carbon atom in the carbonyl group makes the carbon atom an easier target for the nucleophilic attack.
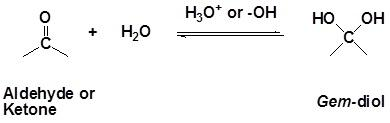
- In case of the acid catalysed nucleophilic reaction where the carbonyl compound is activated, it begins with the protonation (addition of proton) of the carbonyl oxygen by the hydronium ion to form the conjugate acid of carbonyl compound.
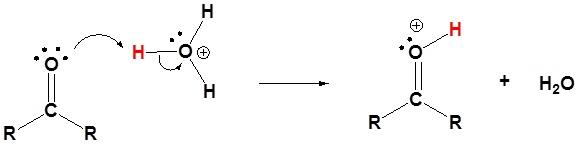
The above step is followed by the attack of the water molecule on the carbon in the carbonyl group on the conjugate acid carbonyl compound formed.
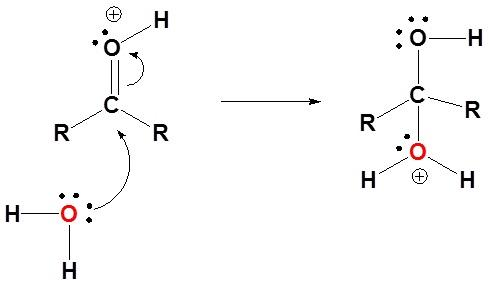
After which deprotonation occurs, as another water molecule receives a proton from the oxygen cation formed in the above step and forms a diol(hydrate).
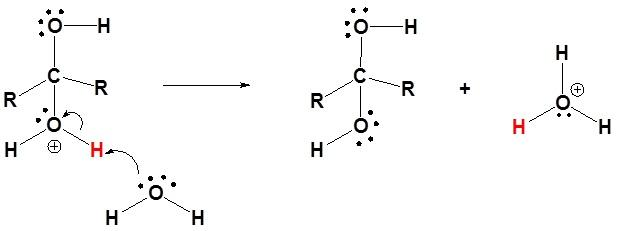
- Whereas, in case of base catalysed nucleophilic reaction the nucleophile is activated, where the hydroxide ion attacks the carbon in the carbonyl group and then the water donates proton to the oxygen anion producing diol(hydrate) and hydroxide.
Step 1: Nucleophilic attack of the hydroxide on the carbon of the carbonyl group, forming alkoxide ion.
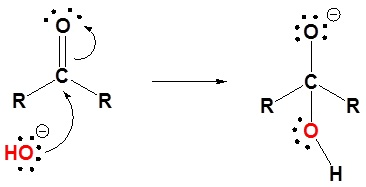
Step 2: Protonation of alkoxide as it gains a proton from the water molecule.
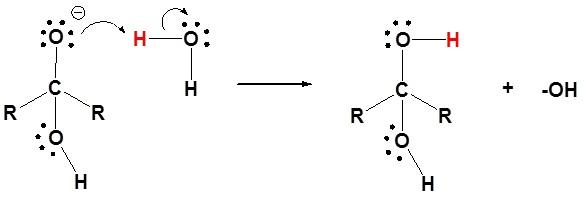
Thus, in case of aldehyde and ketone consisting of the carbonyl group, the nucleophilic addition of water takes place with ease. Therefore, both aldehyde (formaldehyde and acetaldehyde) and ketone(acetone) are soluble in water.
Additional information: The mechanism is catalysed by an acid or base as it speeds up the reaction without affecting the equilibrium. In base catalysed reactions, hydroxide is a better nucleophile than water. Whereas in acid catalysed reaction, the protonated carbonyl is extremely electrophilic making the attack of water as a nucleophile easy.
Note: The extent of solubility further depends on the stability of the carbonyl group and the steric effect (that is, the crowding of the carbonyl group).
- The carbonyl group with highly substituted alkyl groups will be more stable (that is, lower in energy) and thus less prone to hydration. The ketone is therefore less hydrated compared to aldehyde.
- Also, carbonyl groups with more substituents with larger size hinders the nucleophilic attack, leading to decrease in the amount of hydration.
