Question
Question: Which of the following compounds are gem-dihalides? A.Ethylidene chloride B.Ethylene dichloride ...
Which of the following compounds are gem-dihalides?
A.Ethylidene chloride
B.Ethylene dichloride
C.Methylene chloride
D.Benzyl chloride
Solution
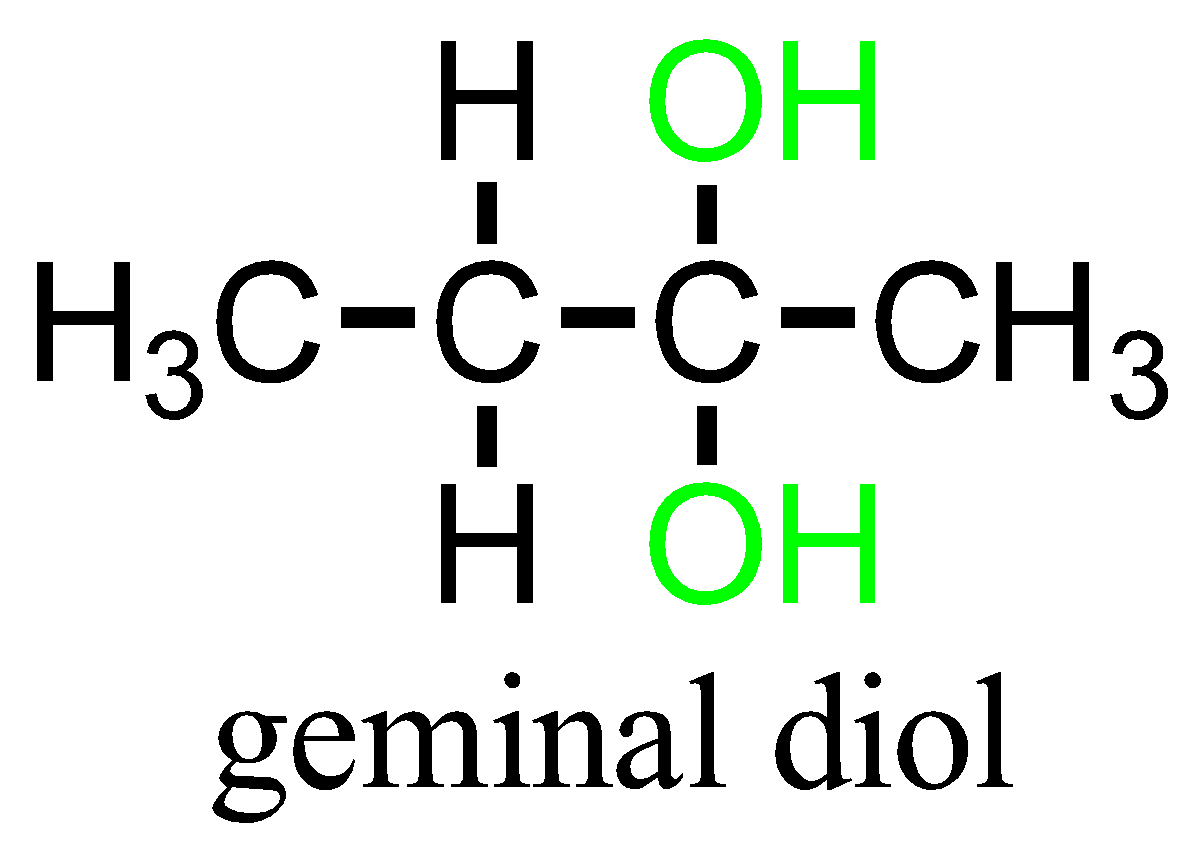
Geminal or Gem dihalides are those compounds in which halides are substituted on the same carbon atom. In other words, they have 1,1 relationships. The term ‘hominal’ can also be used instead of gem-dihalides.
Complete step by step answer:
Geminal dihalides are the one where the two halide groups are located on the same carbon atom maintaining a 1,1 relationship.
Ethylidene chloride is also known as 1,1 Dichloroethane. It has 2 Chlorine atoms on the same carbon atom thus has 1,1 relationship. Thus, it is geminal dihalide.
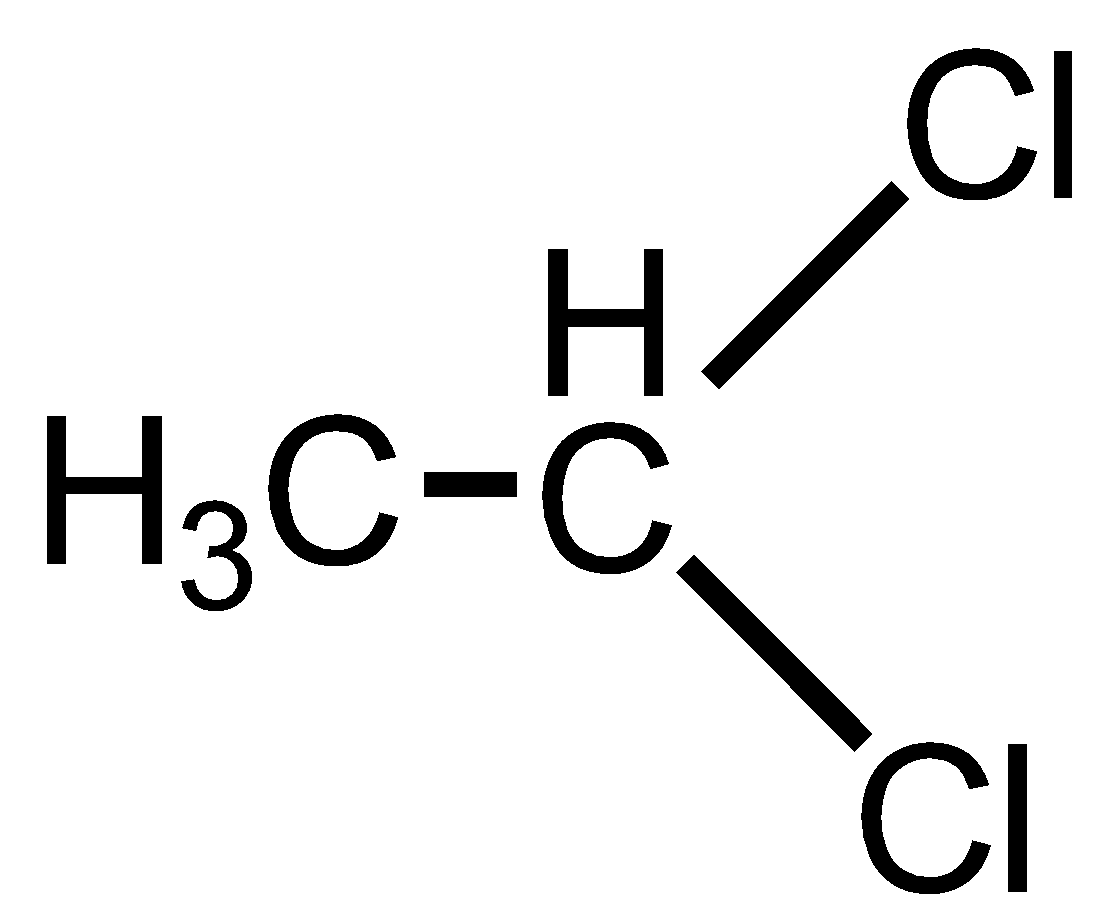
Ethylene dichloride is also known as 1,2 Dichloroethane. It has 2 Chlorine atoms on the different carbon atoms that are adjacent carbon atoms having a 1,2 relationship. Thus, it is not a geminal dihalide.
Methylene chloride is also known as 1,1 Dichloro methane. It has 2 Chlorine atoms on the same carbon atom thus has 1,1 relationship. Thus, it is geminal dihalide.
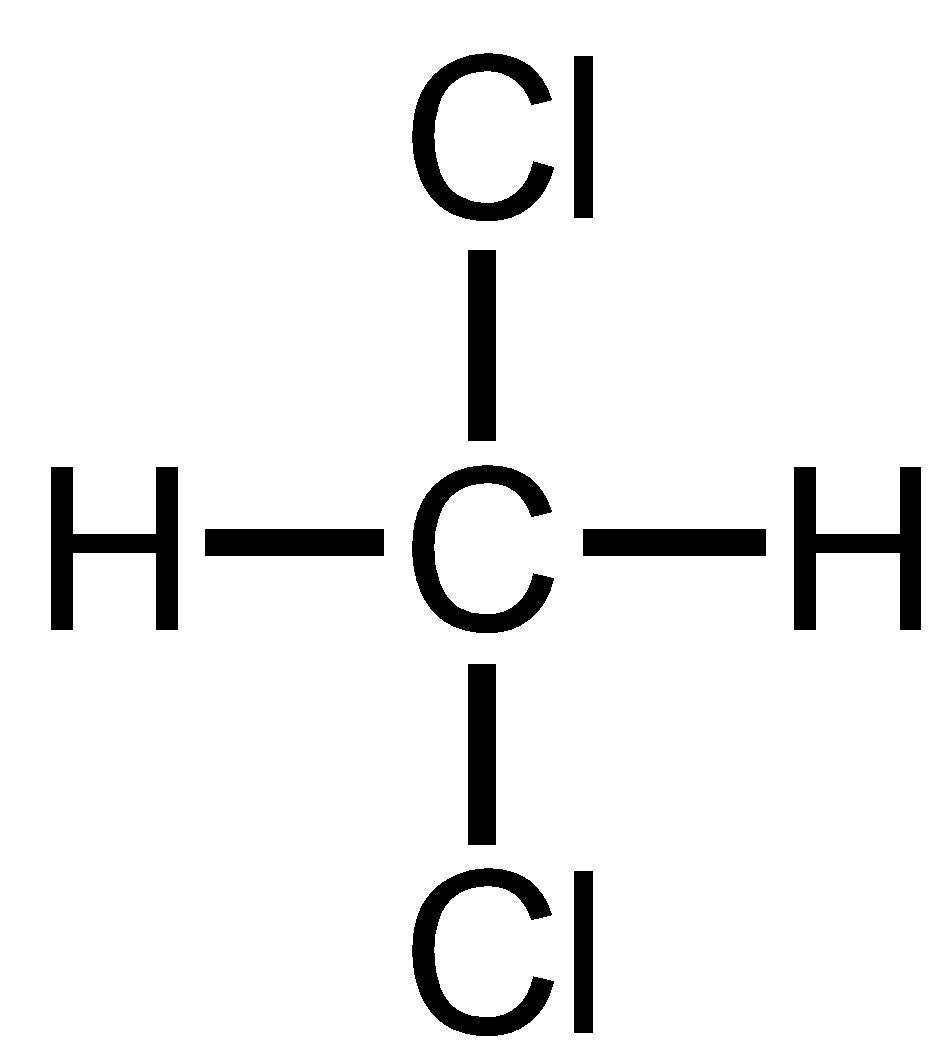
Benzyl chloride has only one chloride atom so there are no dihalides thus ‘gem’ term cannot be prefixed.
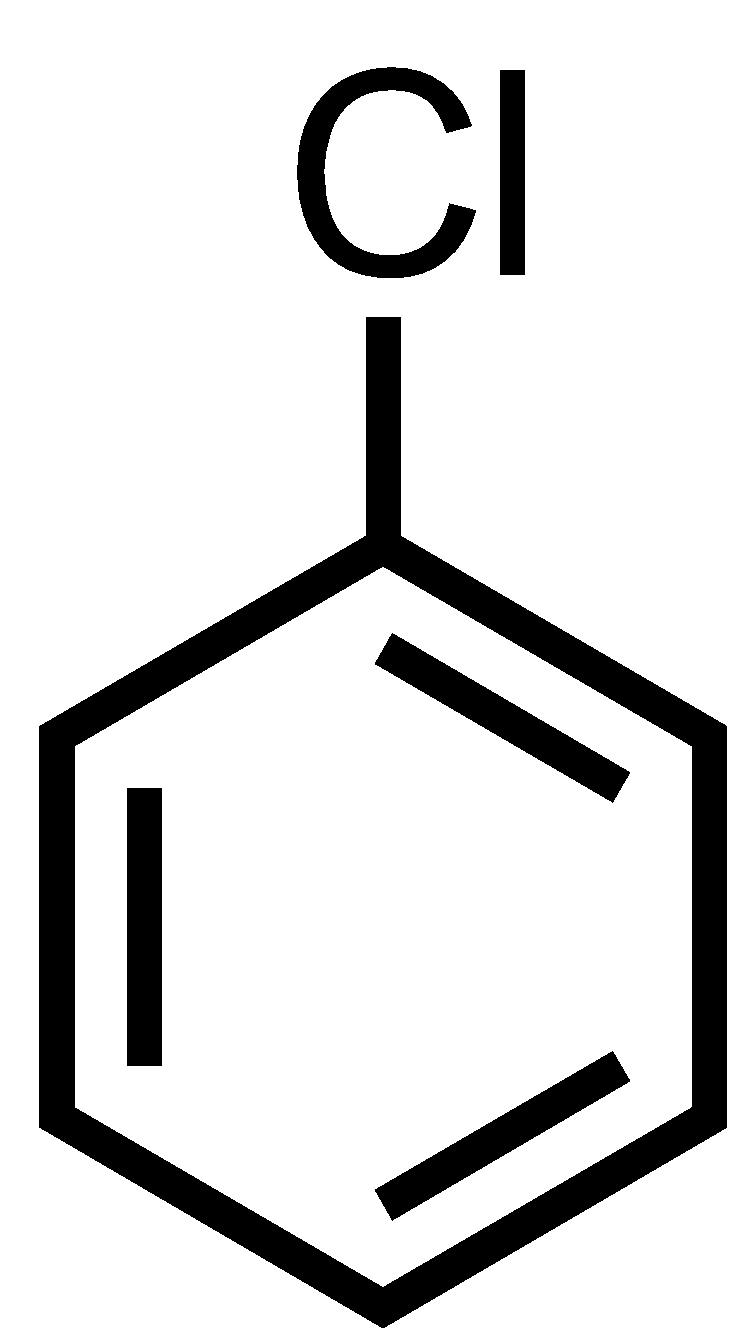
So, the correct options are A and C.
Note:
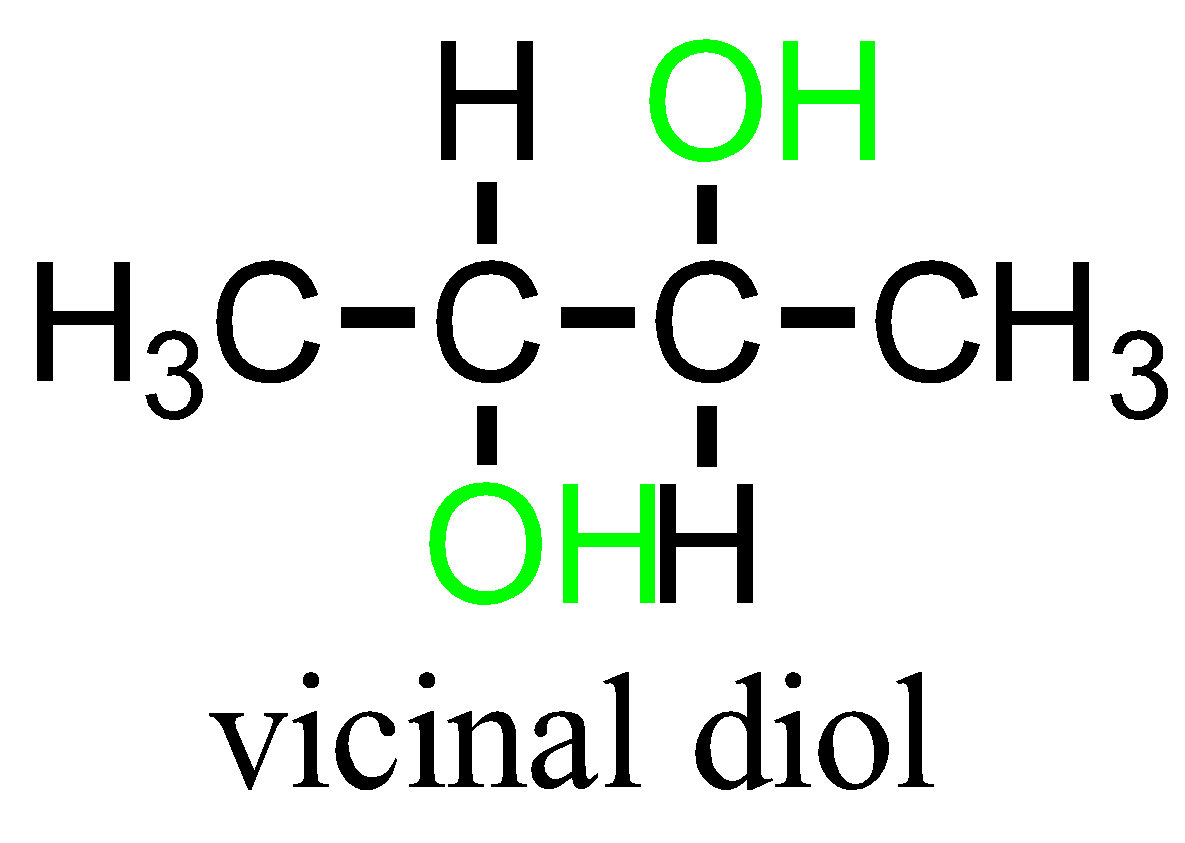
The other common prefix used for mentioning the substituents is “vicinal”. The term “vicinal dihalides” is used for compounds containing two halides on the adjacent carbon atoms. It has 1,2 relationships.

The term “vicinal” and “geminal” can be applied to any substituents that repeat twice. Example: hydroxy groups namely Diols containing compounds.