Question
Question: Which of the following compounds are called unsaturated compounds- that is, it/they add \({{H}_{2}}\...
Which of the following compounds are called unsaturated compounds- that is, it/they add H2 catalytically at ordinary temperature?
(A)
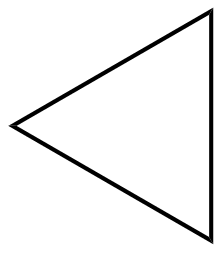
(B)
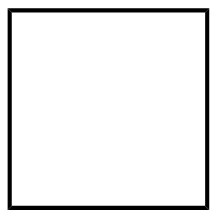
(C)
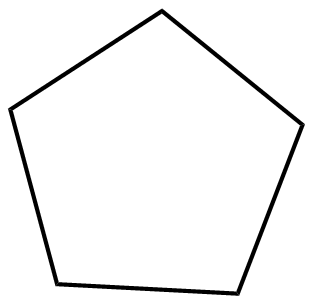
(D)
Solution
Unsaturation can be due to many reasons and not just because of double bond. Apply the formula given below the degree of unsaturation in the above compounds.
Formula: D.U=22C+2+N−H−X
Where,
C = Number of carbon atoms,
N = Number of nitrogen atoms,
H= Number of hydrogen atoms,
X = Number of halogen atoms.
Complete step by step answer:
Degree of Unsaturation also referred to as the index of hydrogen deficiency(IHD) is a measure of the number of rings and pi bonds present in the organic molecule.
However, unsaturation can be due to the presence of atoms like oxygen, nitrogen as well.
We will now calculate the degree of unsaturation for the options and also find whether the molecule is unsaturated or not.
Degree of unsaturation for option (A):
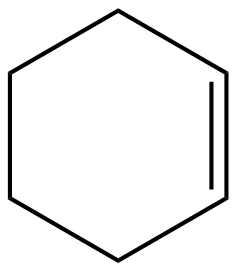
D.U=212+2−10=2
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 2.
Degree of unsaturation for option (B):
D.U=26+2−6=1
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 1.
Degree of unsaturation for option (C):
D.U=28+2−8=1
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 1.
Degree of unsaturation for option (D):
D.U=210+2−10=1
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 1.
So, the correct answer is “Option A,B,C and D”.
Note: Unsaturation in an organic molecule can be due to the following reasons:
-Presence of a double bond (D.U=1)
-Presence of a triple bond (D.U=2)
-Presence of a C=O bond (D.U=1)
-Presence of a C=N bond (D.U=1)
-Presence of a ring (D.U=1)
