Question
Question: Which of the following compounds are aromatic. A. 
B. 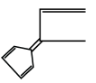
C. 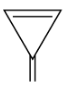
D. 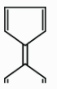
Solution
Aromatic compounds are defined as characterized by the presence of one or more ring which are uniquely stable structures which means the π(pi) electrons of molecules have strong bonding arrangements. The compound must follow these four conditions which are
-The aromatic compound must be a cyclic compound.
-The aromatic compound has planarity i.e. sp2 hybridization.
-Delocalization of π electrons are also present in aromatic compounds.
-The aromatic compound followed the Huckel’s rule of aromaticity i.e. (4n+2)π electrons.
Complete answer:
So, here in option (A), It is a cyclic compound and also has sp2 hybridization in which planarity is present. There is 5π bond that is present in which 5×2πelectrons are involved in the ring.
According to Huckel’s rule of aromaticity i.e. (4π+2) π electron …… (i)
Put the value of n in the equation (i)
In which, n=0→[4(0)+2]=2πelectron pair
n=1→[4(1)+2]=6π electron pair
n=2→[4(2)+2]=10π electron pair
Here 10π electrons are matched to the value of π electron of this compound. So, this compound is an aromatic compound.
In the option (B), the given compound is cyclic compound and have sp2 hybridization, the delocalization of π electrons are also present and 4π bond occur which means 4×2πelectron =8π electrons. According to Huckel’s rule of aromaticity i.e. (4n+2)πelectrons are not applied in this compound due to the value of Huckel’s rule are not equal or match with the 8π electron. So, this is not an aromatic compound.
In the option (C), these are not cyclic compound and sp2 hybridization is absent, delocalization of π electron may occur in which 2π bond and these have 2×2π electron which means 4π electron and their value is not equal to the Huckel’s rule of aromaticity. So, this compound does not follow the conditions of aromaticity.
In the option (D), these are cyclic compounds and satisfy all conditions of aromaticity except one of them i.e. these do not follow the Huckel’s rule of aromaticity. The second five membered cyclic ring has an anion charge not cation. So, it loses aromaticity due to 8π electrons, it is not an aromatic ring.
Therefore, (A) is the correct option because this compound is an aromatic compound which follows all the properties of aromaticity.
Note:
Huckel’s rule of aromaticity is the rule which determines that the ring is planar which has aromatic properties in the molecule. The cyclic molecules are considered aromatic if it has 4n+2π electrons.
