Question
Question: Which of the following compounds are antiaromatic? 
Solution
A compound is said to the anti-aromatic, if it is cyclic, planar, have the hybridization as sp2and obeys the(4n)πes−rule in which n can be any integer such as 1,2,3,---and so on. From the above compounds, which satisfy this condition ,is called the anti -aromatic compound. now identify it.
Complete step by step answer:
First of all, we should know what aromatic compounds are. A compound is said to be aromatic if it satisfies the following conditions:
1. there should be (4n+2)πes− and here, n can be any integer 1,2,3,3-----and so on.
2. Compounds should be cyclic( i.e. in ring) and must contain unhybridized p-orbitals and the ring should be planar .
3. All the carbon atoms should be sp2 hybridized.
4. Cyclic systems should have conjugation ( i.e. there should be the delocalization of pi-electrons).
On the other hand, anti-aromatic compounds also satisfy all the conditions except the one, that there are (4n)πes− instead of (4n+2)πes−. And the pi electrons are in the multiple of 4 like
4,8,12,16---and so on.
| S.no | Compound | Cyclic | Planar | sp2hybridization | (4n)πes− | Anti -aromatic |
|---|---|---|---|---|---|---|
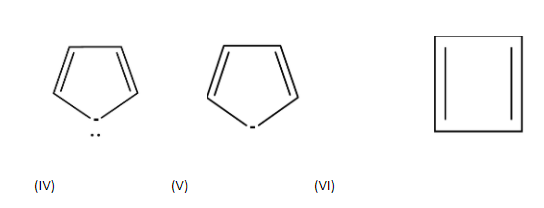
| 1. | 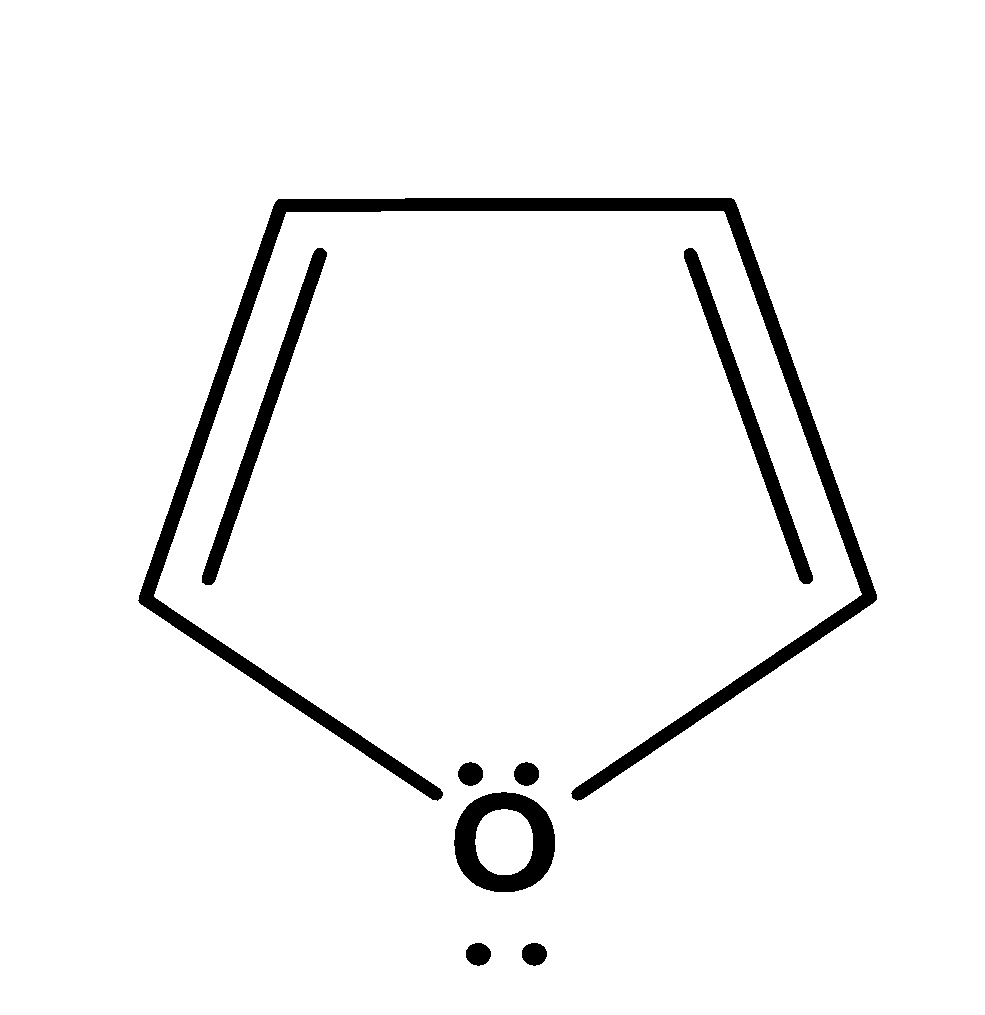 | cyclic | planar | sp2 | Has 2 pi bonds i.e. 4 electrons (n=1) and 2 electrons of the lone pair which is inside the plane i.e. there are a total no of 6 electrons . so, it doesn’t obey this rule. | no |
| 2. | 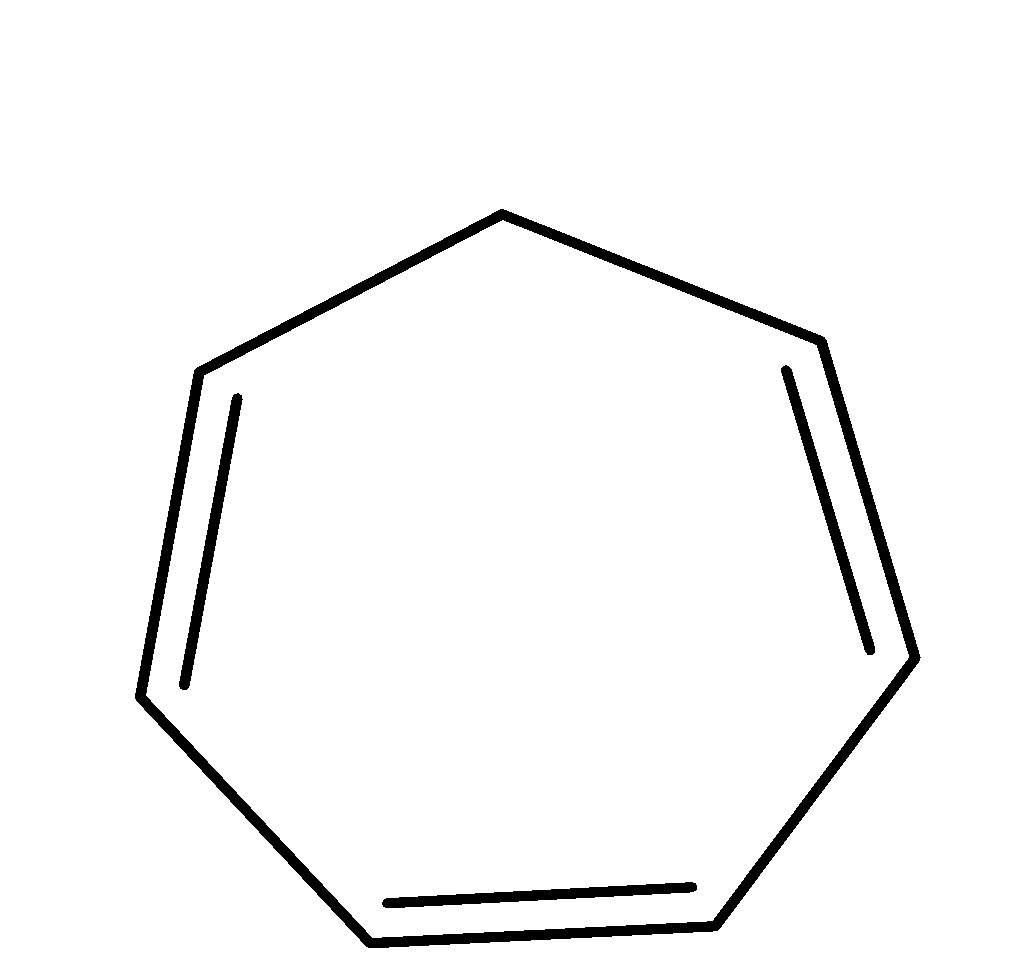 | cyclic | planar | One carbon is sp3hybridized | Has 3 pi bonds i.e. 6 electrons=1 and it is not a multiple of 4 and doesn’t obey this rule. | no |
| 3. | 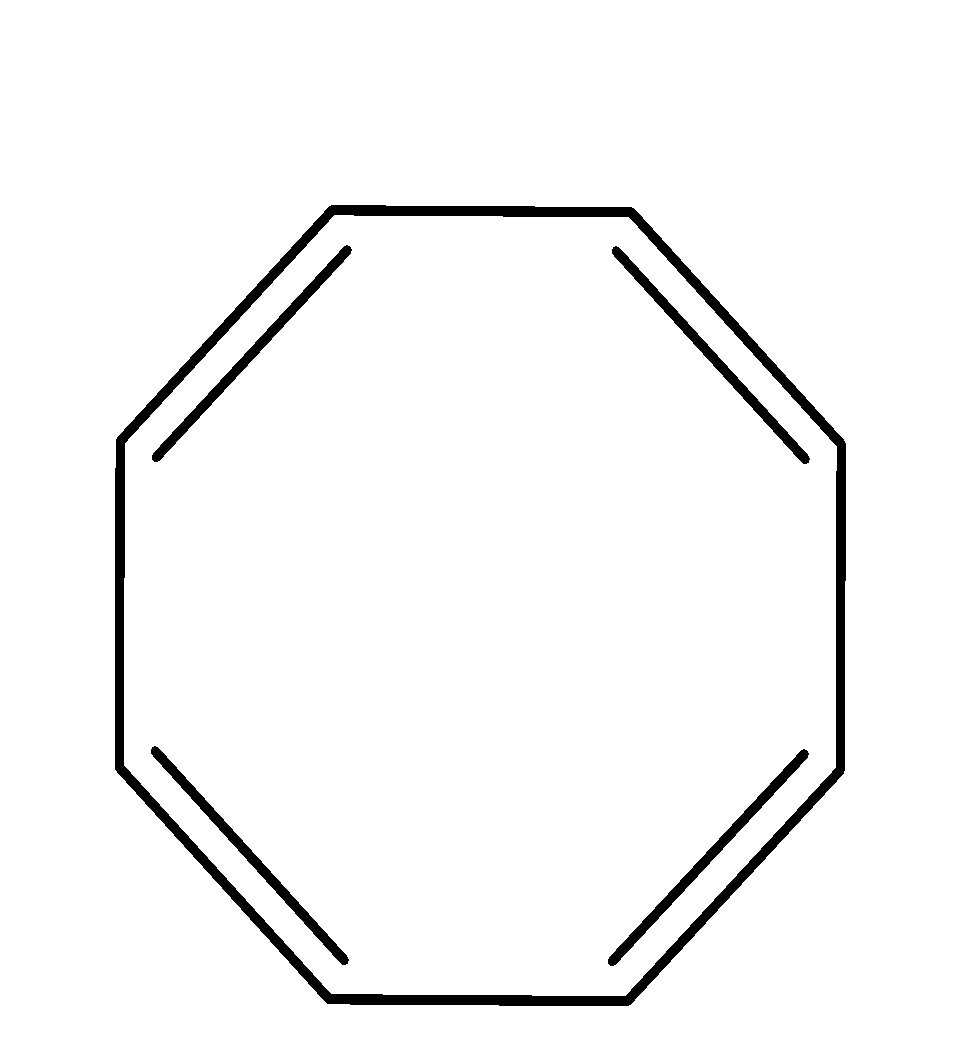 | cyclic | planar | sp2 | Has 4 pi bonds i.e.8 electrons(n=2) and is multiple of 4. So, it obeys this rule. | yes |
| 4. | 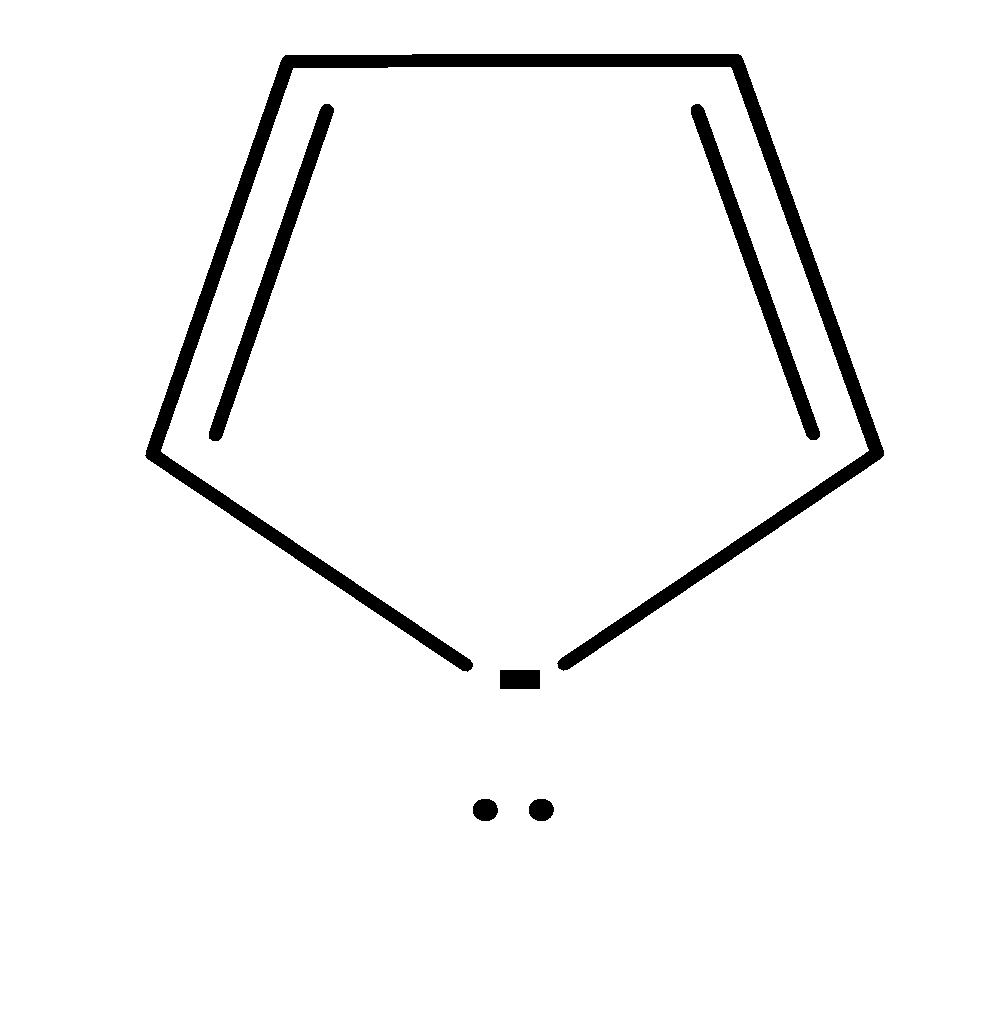 | Cyclic | planar | sp2 | Has 2 pi bonds i.e. 4 electrons (n=1) and 1 electron of the negative charge and electrons of lone pair would not be considered because they are outside the plane i.e. there are a total no of 3 electrons . so, it doesn’t obey this rule. | no |
| 5. | 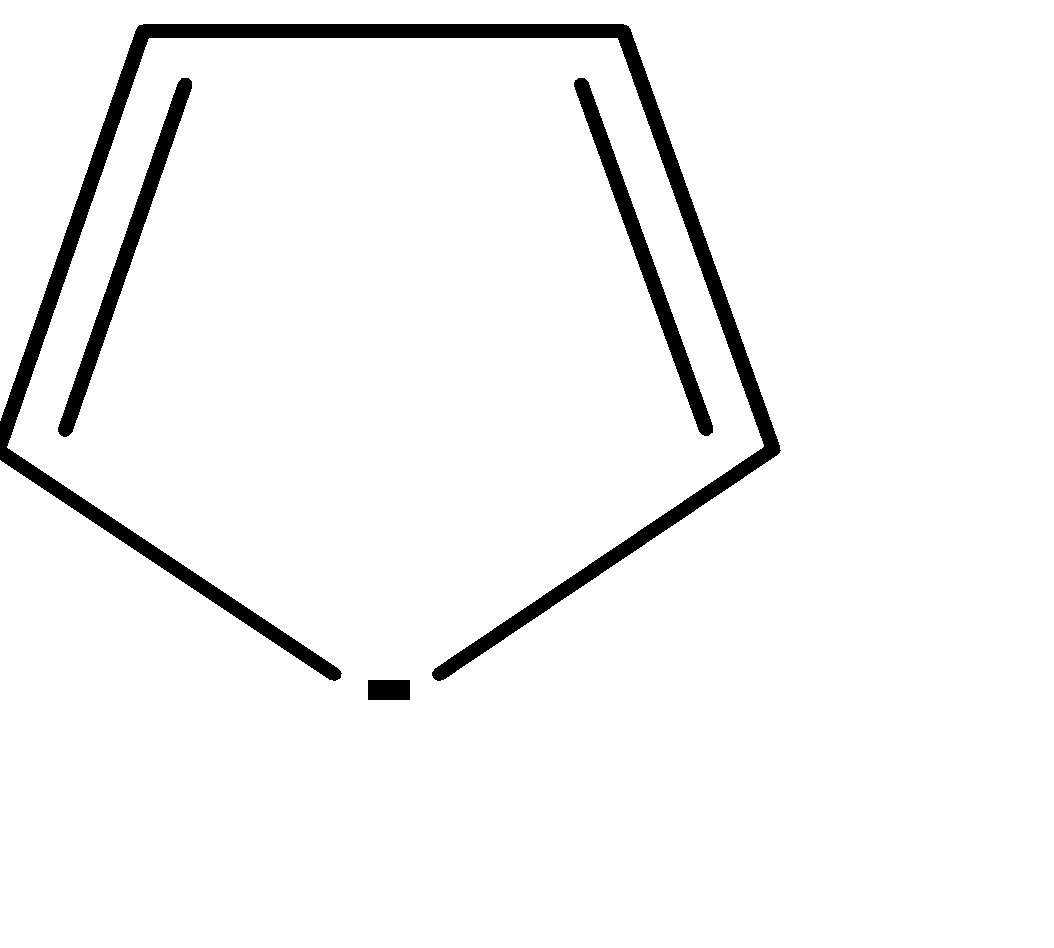 | Cyclic | planar | sp2 | Has 2 pi bonds i.e. 4 electrons (n=1) and 2 electrons of the lone pair i.e. there are a total no of 6 electrons . so, it doesn’t obey this rule. | yes |
| 6. | 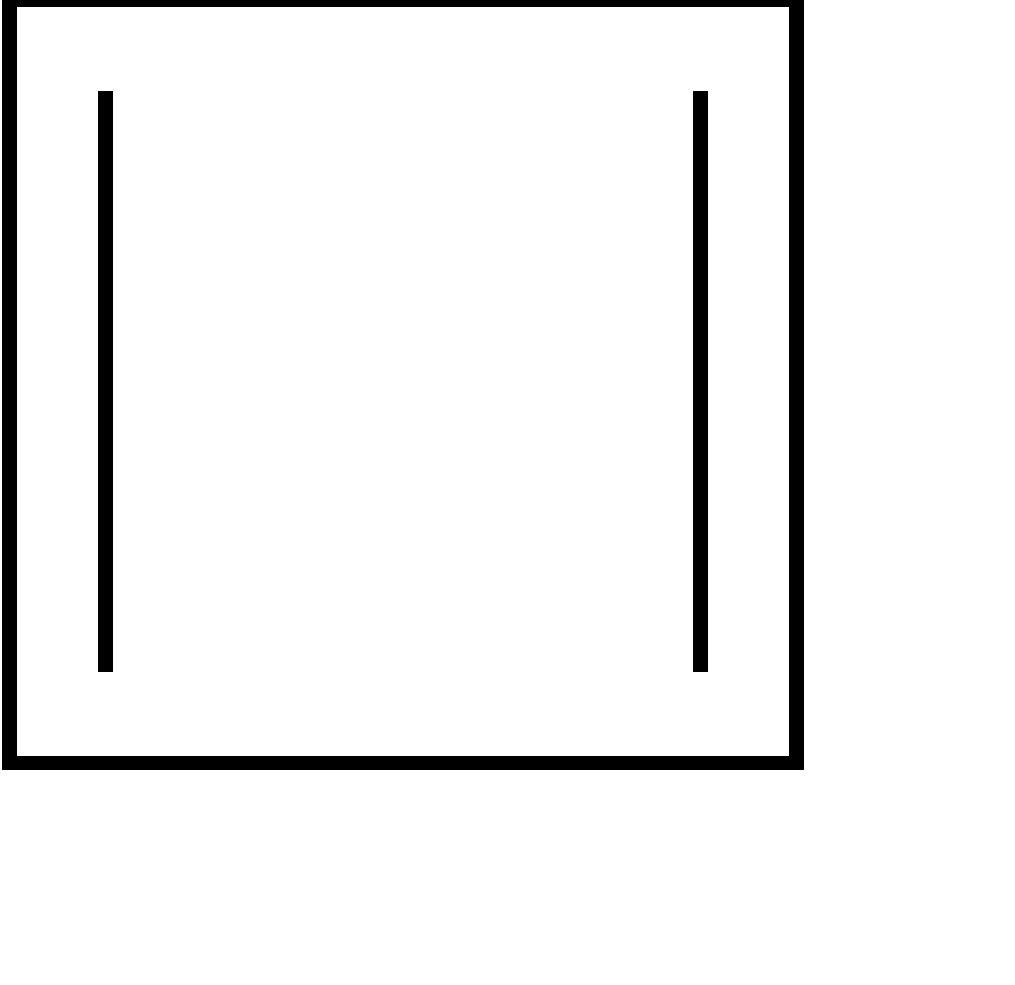 | cyclic | planar | sp2 | Has 2 pi bonds i.e. 4 electrons (n=1) and is multiple of 4. So, obey this rule. | yes |
Note: in this, formula (4n)πes−, the value of cannot be negative and it always has a positive value and the compounds which doesn’t above any conditions or any one condition of the aromaticity, those compounds are said to be non-aromatic compounds.
