Question
Question: Which of the following compound will give \[{{\text{S}}_{\text{N}}}1{\text{ and }}{{\text{S}}_{\text...
Which of the following compound will give SN1 and SN2 reactions with considerable rate?
I.C6H5−CH2−Br
II.CH2=CH−CH2−Br
III.CH3−CH(Br)−CH3
IV.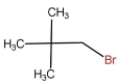
Select the correct answer according to the given codes below.
A.I, II and III
B.I, II and IV
C.II, III and IV
D.I, III and IV
Solution
The secondary alkyl halide reacts equally with the SN1 and SN2 reaction. If the carbocation is stabilised by the resonance then SN1 is more preferred.
Complete step by step answer:
The conversion of haloalkanes to alcohol is done by SN1 and SN2 reactions using hydroxide ion as reagent in aqueous medium.
SN1 reaction is also known as nucleophilic substitution unimolecular reaction. It includes two steps. Tertiary carbocations are most stable and hence the tertiary alkyl halides are more reactive towards SN1 reactions followed by secondary and primary carbocation.
SN2 reaction is also known as nucleophilic substitution bimolecular reaction. It is a one step reaction and no intermediate forms in between. Hence, the rate of reaction depends upon the steric hindrance. Since primary alkyl halides are least sterically hindered and hence they are very much reactive towards the SN2 reaction followed by secondary and primary.

The above molecule is a typical tertiary alkyl halide and hence will always go for SN1 reaction because of the formation of stable carbocation.
CH3−CH(Br)−CH3
This is secondary alkyl halide and hence it will react equally with both the mechanism and have almost the same rate toward both.
CH2=CH−CH2−Br
C6H5−CH2−Br
No doubt both of them are primary alkyl halide and should for SN2 reaction mechanism because of low steric hindrance, but the carbocation formed is stabilised by the resonance of phenol group and alkene. So they both will react at considerable rate with SN1 and SN2 mechanism.
Hence, the correct option is A.
Note:
Rate of reaction in case of SN1 depends only on haloalkane, not on nucleophiles. Rate of SN2 reaction depends upon the, nature of the solvent, structure of the substrate, nature of the nucleophile and Effect of leaving-group.
