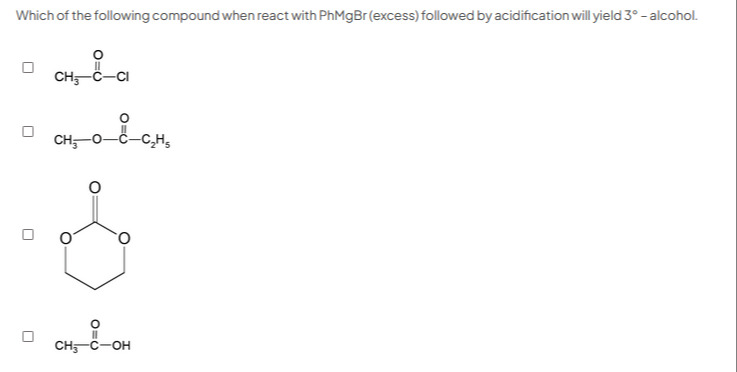
Question
Question: Which of the following compound when react with PhMgBr (excess) followed by acidification will yield...
Which of the following compound when react with PhMgBr (excess) followed by acidification will yield 3° - alcohol.
□ CH3-C-CI
□ CH3-O-C-C2H5
□
□ CH3-C-OH
CH3-C-CI
CH3-O-C-C2H5
1,3-dioxan-2-one
CH3-C-OH
CH3-C-CI, CH3-O-C-C2H5, 1,3-dioxan-2-one
Solution
The reaction of Grignard reagents with carbonyl compounds is a key reaction in organic chemistry. When a Grignard reagent reacts with an acid derivative (acid halide, ester, anhydride), the first step is nucleophilic addition to the carbonyl carbon, followed by elimination of the leaving group to form a ketone or aldehyde. If excess Grignard reagent is present, the ketone or aldehyde further reacts with the Grignard reagent via nucleophilic addition to form an alkoxide, which upon acidification yields an alcohol.
-
Acetyl chloride (CH3COCl): Acetyl chloride is an acid halide. Reaction with PhMgBr (excess): CH3COCl+PhMgBr→CH3COPh (acetophenone) + MgBrCl CH3COPh+PhMgBr→CH3-C(OMgBr)(Ph)2 Acidification: CH3-C(OMgBr)(Ph)2+H3O+→CH3-C(OH)(Ph)2 (2-phenyl-2-propanol). This is a tertiary alcohol, as the carbon bearing the -OH group is bonded to one methyl group and two phenyl groups.
-
Ethyl acetate (CH3COOC2H5): Ethyl acetate is an ester. Reaction with PhMgBr (excess): CH3COOC2H5+PhMgBr→CH3COPh (acetophenone) + MgBr(OC2H5) CH3COPh+PhMgBr→CH3-C(OMgBr)(Ph)2 Acidification: CH3-C(OMgBr)(Ph)2+H3O+→CH3-C(OH)(Ph)2 (2-phenyl-2-propanol). This is a tertiary alcohol.
-
1,3-Dioxan-2-one (Trimethylene carbonate): This is a cyclic carbonate, which is a diester of carbonic acid. Reaction with PhMgBr (excess): Initial attack of PhMgBr on the carbonyl carbon opens the ring.
Elimination of an alkoxy group opens the ring, forming an intermediate. Let's say the bond between the carbonyl carbon and one of the ring oxygen atoms breaks.
This is a ketone with an alkoxide group: PhCO(CH2)3OMgBr. Since PhMgBr is in excess, it reacts with the ketone: PhCO(CH2)3OMgBr+PhMgBr→Ph-C(OMgBr)(Ph)-(CH2)3OMgBr Acidification: Ph-C(OMgBr)(Ph)-(CH2)3OMgBr+2H3O+→Ph-C(OH)(Ph)-(CH2)3OH + 2MgBr++2H2O The resulting alcohol is 4-hydroxy-1,1-diphenylbutan-1-ol. The carbon bearing the first -OH group is bonded to two phenyl groups and a -(CH2)3OH group. This is a tertiary alcohol.
-
Acetic acid (CH3COOH): Acetic acid is a carboxylic acid. The first reaction with PhMgBr is an acid-base reaction: CH3COOH+PhMgBr→CH3COO−MgBr++PhH The carboxylate salt is generally unreactive towards Grignard reagents under normal conditions. Therefore, no alcohol is formed.
Based on the reactions, acetyl chloride, ethyl acetate, and 1,3-dioxan-2-one all yield a tertiary alcohol when reacted with excess PhMgBr followed by acidification.
