Question
Question: Which of the following compound(s) will give dimethyl glyoxal with \[Se{0_2}\] ? A. Acetone B ...
Which of the following compound(s) will give dimethyl glyoxal with Se02 ?
A. Acetone
B Acetophenone
C Ethyl methyl ketone
D Propionaldehyde
Solution
A chemical equation occurs when the number of atoms involved in the reactant's side is equal to the number of atoms on the product side. A reagent can be a substance or mixture of compounds which are used in chemical analysis or reactions. When a reagent is added to a system it causes chemical reactions. For a particular reaction, a particular reagent gets consumed in the process of the chemical reaction.
Complete step by step answer:
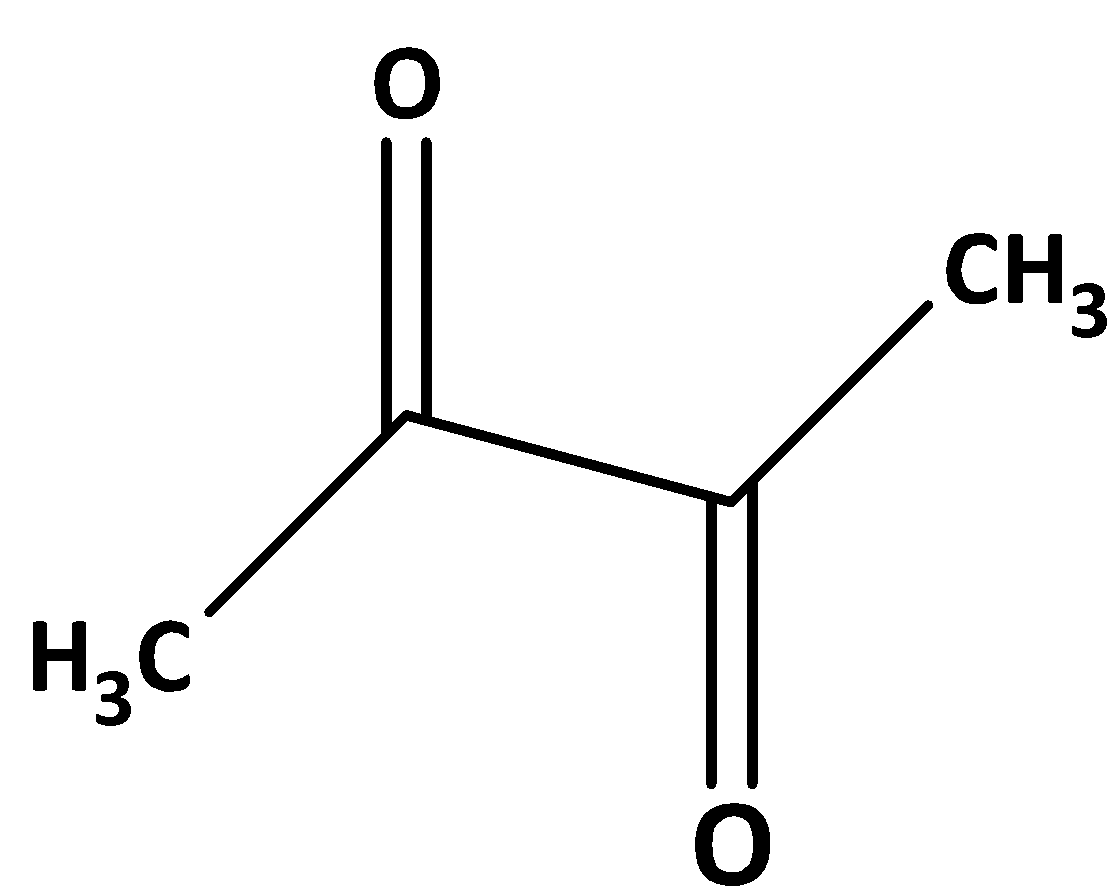
The structure of dimethyl glyoxal is shown below.
To answer this question the uses of reagents in organic chemistry should be known. Like this oxidizes the alpha carbon of the carbonyl group. Therefore, dimethyl glyoxal can be prepared by using a carbonyl compound.
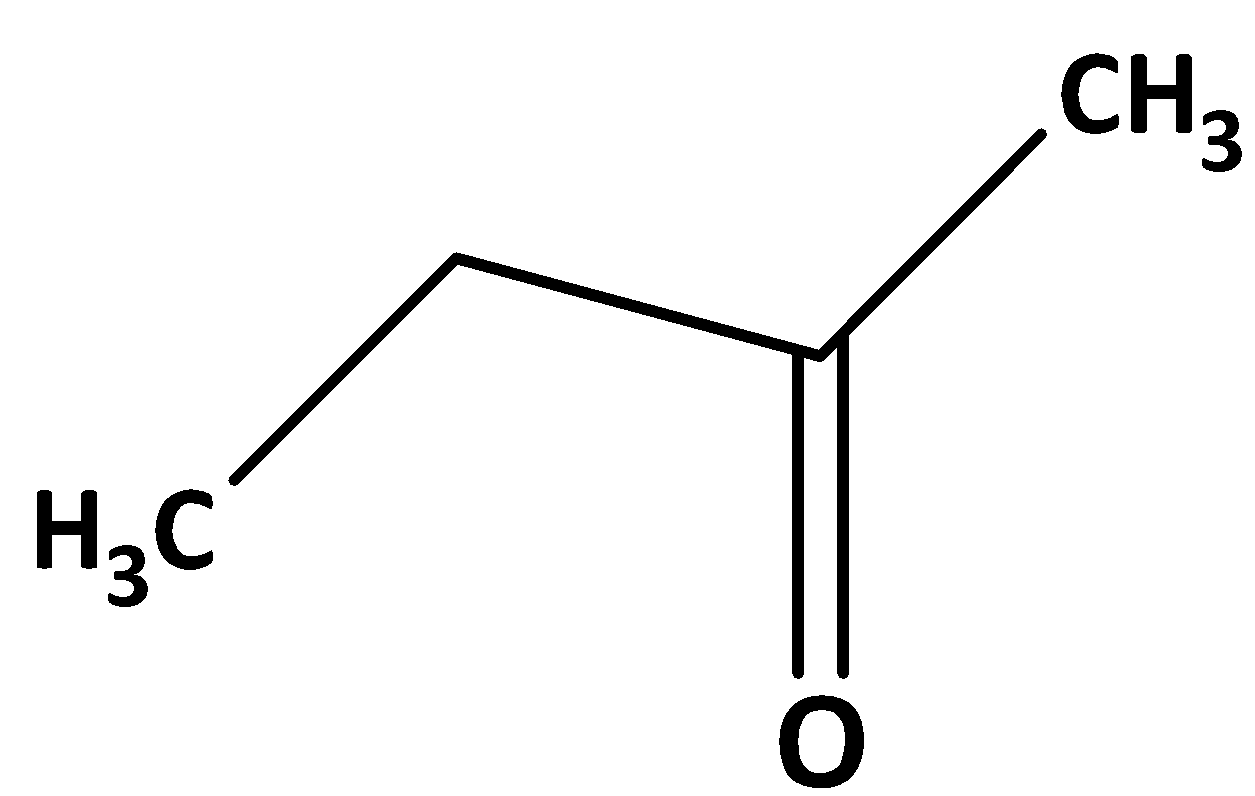
Now the answer is Ethyl methyl ketone. The structure is shown below,
The conversion is shown below.
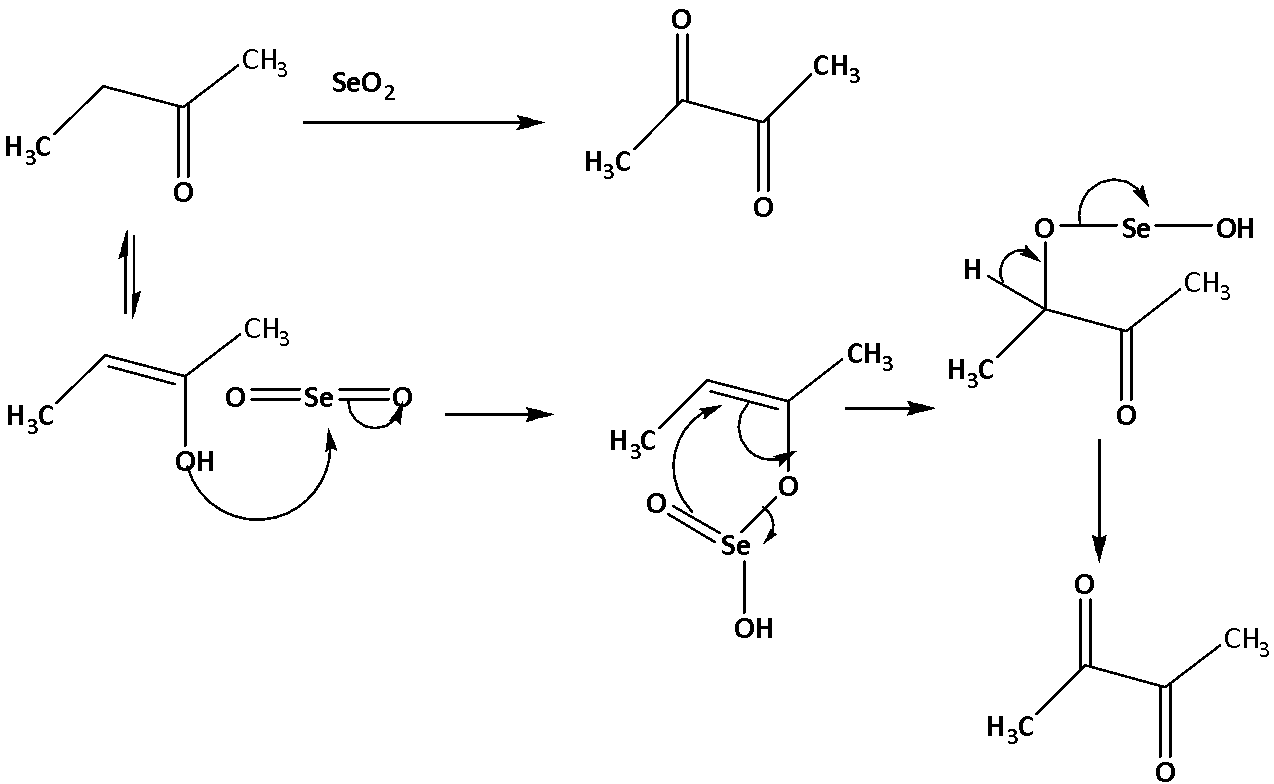
So, the correct answer is C.
Note:
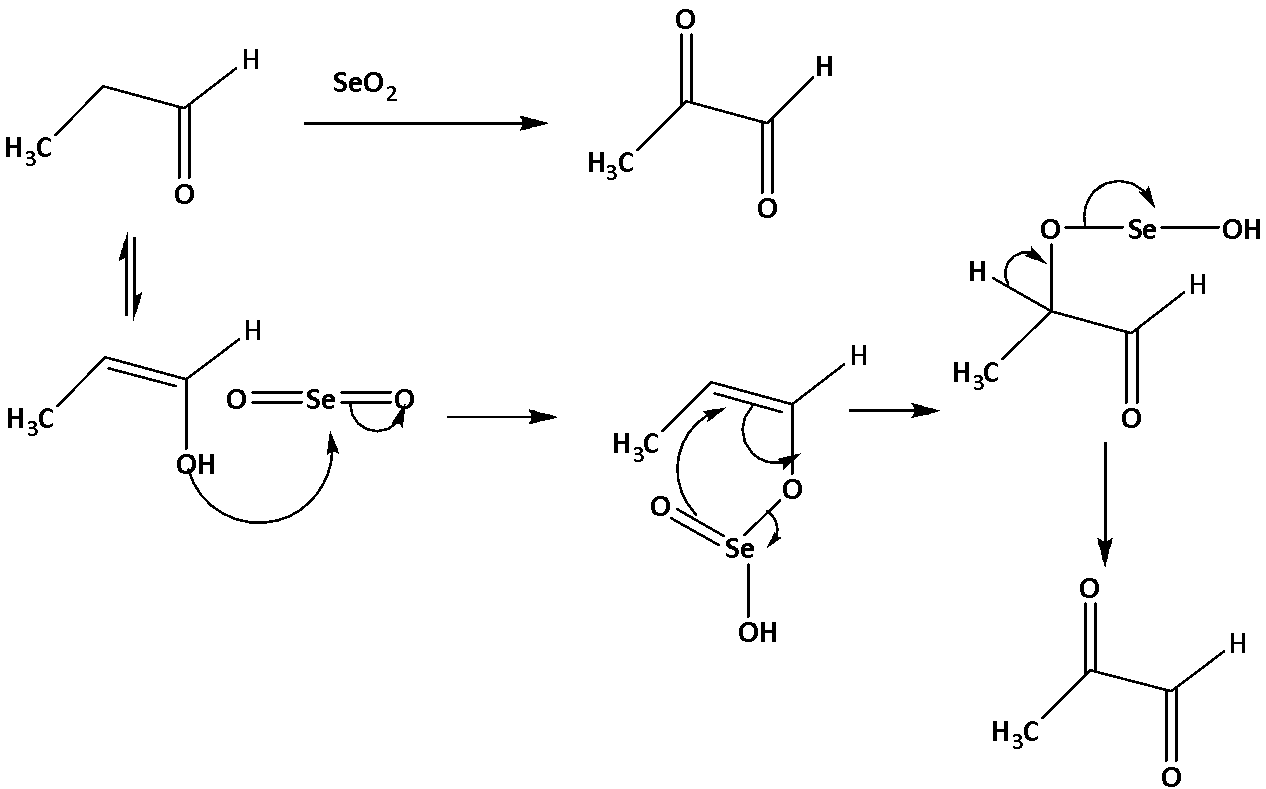
In the case of propionaldehyde, it also contains the alpha carbon of a carbonyl group and can be oxidized by . But it will not produce the dimethyl glyoxal, the reaction is shown below.