Question
Question: Which of the following compound have \({\text{X}} - {\text{O}} - {\text{X}}\) linkage where ‘\({\tex...
Which of the following compound have X−O−X linkage where ‘X’ is the so called central atom like P, S etc?
(A) P2O84−
(B) S2O32−
(C) γ−SO3
(D) S2O52−
Solution
To solve this we must first draw the structures of all the four given compounds. From the structures we can determine the compound having X−O−X linkage. First of all we should draw the exact structure of the compound to find out the central atom.
Complete Step by step answer: P2O84− is known as peroxydisulfate ion. Draw the structure of P2O84−:
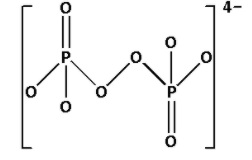
In the structure of P2O84−, we cannot see P−O−P linkage. Thus, P2O84− does not have X−O−X linkage.
Thus, option (A) is not correct.
S2O32− is known as thiosulphate ion. Draw the structure of S2O32−:
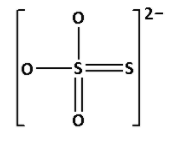
In the structure of S2O32−, we cannot see S−O−S linkage. Thus, S2O32− does not have X−O−X linkage.
Thus, option (B) is not correct.
γ−SO3 is known as sulphur trioxide. Draw the structure of γ−SO3:
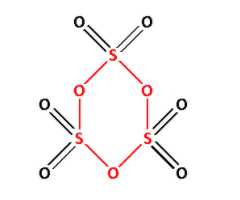
In the structure of γ−SO3, we can see S−O−S linkage. The S−O−S is shown in red colour. Thus, γ−SO3 has X−O−X linkage.
Thus, option (C) is correct.
S2O52− is known as metabisulphite. Draw the structure of S2O52−:
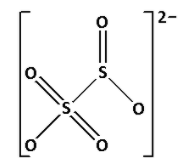
In the structure of S2O52−, we cannot see S−O−S linkage. Thus, S2O52− does not have X−O−X linkage.
Thus, option (D) is not correct.
Thus, the compound have X−O−X linkage where ‘X’ is the so called central atom like P, S etc is γ−SO3.
Thus, the correct option is (C) γ−SO3.
Note: Sulphur trioxide (SO3) has many structural changes and thus, it has many different forms. These changes are caused by the traces of water. γ−SO3 is very pure gaseous sulphur trioxide. It is a trimeric structure i.e. it has three molecules of sulphur trioxide. γ−SO3 forms a cyclic structure. It is colourless solid in appearance. Sulphur trioxide is highly hygroscopic in nature i.e. it can easily attract water molecules from its surroundings by absorption or adsorption.
