Question
Question: Which of the following compound gives benzoic acid when it reacts with hot $KMnO_4$ followed by acid...
Which of the following compound gives benzoic acid when it reacts with hot KMnO4 followed by acidification.
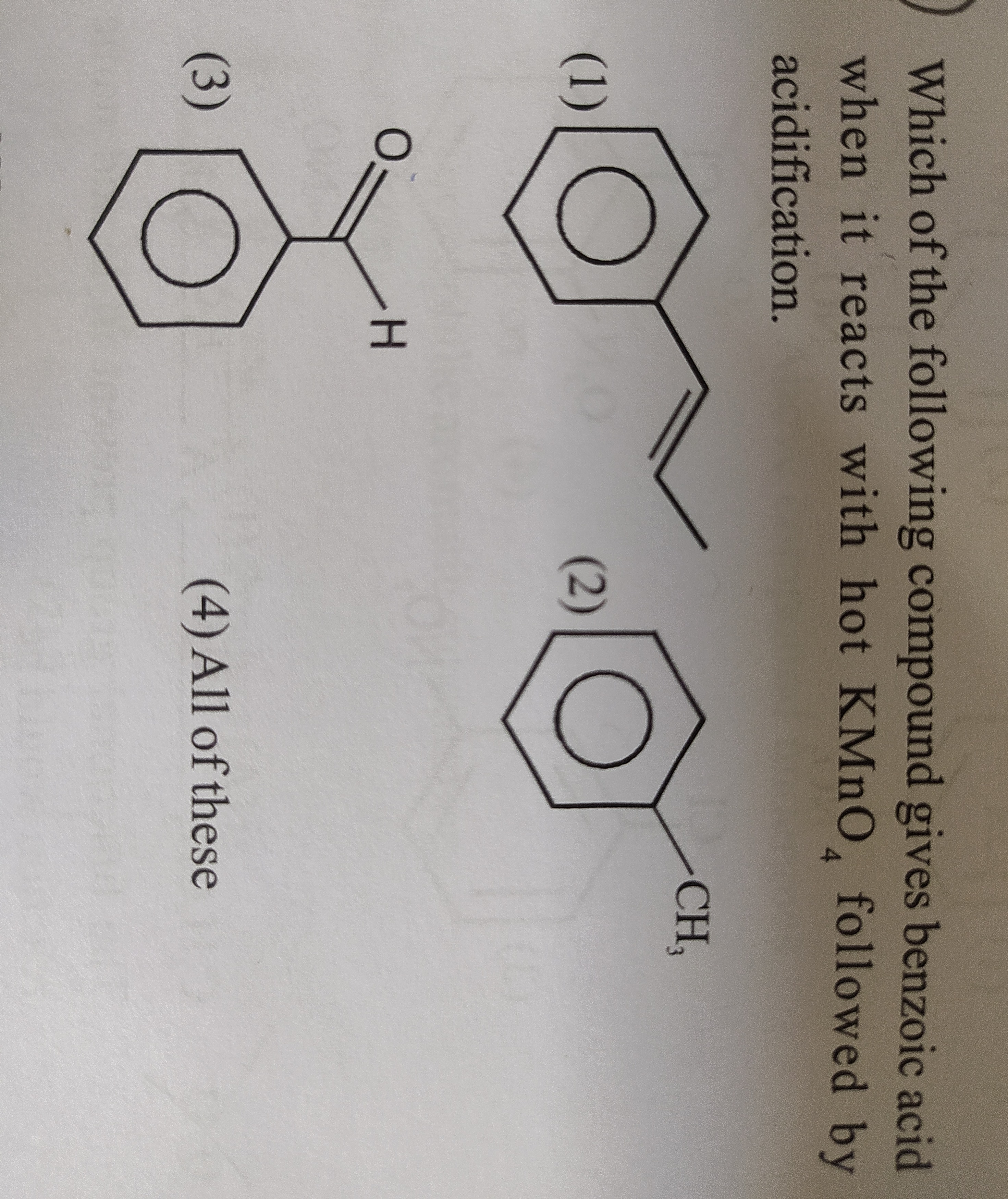
Styrene
Toluene
Benzaldehyde
All of these
All of these
Solution
The reaction of organic compounds with hot potassium permanganate (KMnO4) followed by acidification is a strong oxidation reaction. We need to determine which of the given compounds yields benzoic acid under these conditions.
Option (1): Styrene (Vinylbenzene). The structure is C6H5−CH=CH2. Hot KMnO4 causes oxidative cleavage of the double bond. The carbon atom of the double bond attached to the phenyl ring is oxidized to a carboxylic acid group, and the terminal carbon atom is oxidized to carbon dioxide.
C6H5−CH=CH2KMnO4, heatC6H5−COOH+CO2+H2O
Thus, styrene gives benzoic acid.
Option (2): Toluene (Methylbenzene). The structure is C6H5−CH3. Alkyl groups attached to a benzene ring with at least one benzylic hydrogen are oxidized to a carboxylic acid group by hot KMnO4. The methyl group in toluene has benzylic hydrogens.
C6H5−CH3KMnO4, heatC6H5−COOKH+C6H5−COOH
Thus, toluene gives benzoic acid.
Option (3): Benzaldehyde. The structure is C6H5−CHO. Aldehydes are easily oxidized to carboxylic acids by strong oxidizing agents like KMnO4.
C6H5−CHOKMnO4, heatC6H5−COOKH+C6H5−COOH
Thus, benzaldehyde gives benzoic acid.
Since all three compounds (styrene, toluene, and benzaldehyde) give benzoic acid when reacted with hot KMnO4 followed by acidification, option (4) "All of these" is the correct answer.
