Question
Question: Which of the following complexes is used to be an anticancer agent? (A) mer-\([Co{(N{H_3})_3}Cl]\)...
Which of the following complexes is used to be an anticancer agent?
(A) mer-[Co(NH3)3Cl]
(B) cis-[PtCl2(NH3)2]
(C) cis-K2[PtCl2Br2]
(D) Na2CoCl4
Solution
The complex which is used as an anticancer agent involves a precious metal. This complex has two isomers and one isomer is very highly effective against cancer. The synthesis of this complex involves treatment with ammonia.
Complete answer:
Let’s know which complex is used to cure the cancer.
- Cisplatin is the complex used for the treatment of cancer. The chemical we use in the treatment of cancer is also called an anticancer agent.
- The chemical formula of the Cisplatin is cis-[PtCl2(NH3)2]. Its molecular structure can be given as
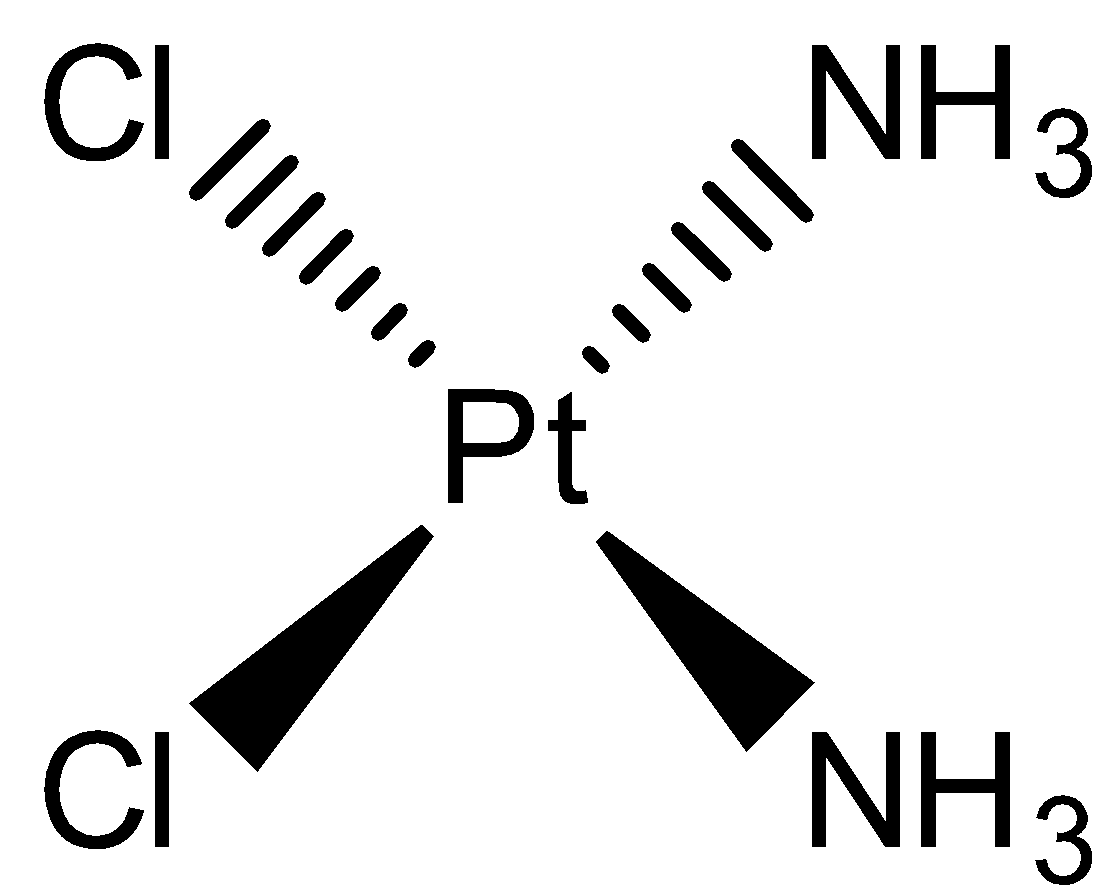
- We can see that this complex is a square planar complex. The oxidation number of Pt metal in this complex is +2.
- It can be obtained from potassium tetrachloroplatinate by allowing it to react with excess KI followed by ammonia. Then the product’s treatment with silver nitrate and exess KI gives cis-[PtCl2(NH3)2] .
- With this anticancer agent we can treat many types of cancers including testicular cancer, breast cancer, ovarian cancer, lung cancer, etc.
- It is given intravenously into our body. That means that it is given by injection into our body.
- Is special use is in testicular cancer. When this drug was not discovered, the cure rate of the patients was 10% but after its discovery, the cure rate rose to 85%.
Thus, we can say that the correct answer is (B).
Note:
Cisplatin interferes with DNA replication and thus it kills the fastest proliferating cells. Generally those cells are cancerous. The trans isomer of this complex does not exhibit required pharmacological effect.
