Question
Question: Which of the following combination will give t-butyl alcohol when treated with Grignard reagent (A...
Which of the following combination will give t-butyl alcohol when treated with Grignard reagent
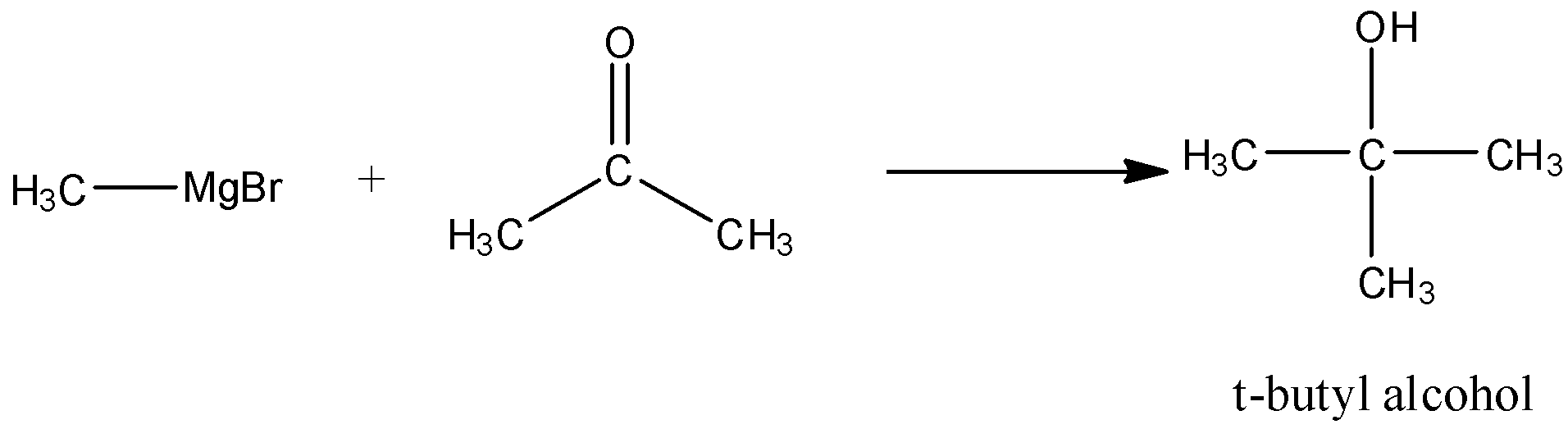
(A) CH3MgBr+CH3COCH3
(B) C2H5MgBr+CH3COCH3
(C) CH3MgBr+(CH3)3COH
(D) CH3MgBr+CH3CH2CH(OH)CH3
Solution
Grignard reagents are an organo-metallic halogen compound which is represented by R-Mg2+X- , where R could be an alkyl or aryl group and X represents the halogen atom.
- The alkyl group of Grignard reagents acts as a strong base and a strong nucleophile. So Grignard reagent shows either acid base reaction or nucleophilic addition reaction and reacts with only those compounds which contain acidic hydrogen or which contains carbonyl group (-C=O).
Complete step by step answer:
- In the presence of carbonyl group Grignard reagent performs nucleophilic addition reaction. Ketone on reacting with Grignard reagent (RMgX) gives alcohol on hydrolysis; hence methyl magnesium bromide reacts with propanone to form t-butylalcohol .
- In the same manner ethyl magnesium bromide reacts with propanone to form t-pentylalcohol.
- In the presence of acidic hydrogen, Grignard reagents perform acid base reactions. Alcohol reacting with Grignard reagent (RMgX) gives hydrocarbons of respective alkyl halide and magnesium alkoxide on hydrolysis. Methyl magnesium bromide gives methane and magnesium alkoxide after reacting with t-butylalcohol . In the same manner methyl magnesium bromide gives methane and magnesium butanoxide after reacting with sec-butylalcohol. The reaction is shown in the following chemical reactions.
Note: Grignard reagents are highly reactive organic compounds and it can react with any source of proton to give hydrocarbons because acid base reactions are much faster than nucleophilic addition reactions. So it is therefore necessary to avoid even trace amounts of moisture from Grignard reagent.
- Acid base reaction is much faster than nucleophilic addition reaction.
