Question
Question: Which of the following carbocations is expected to be most stable? A)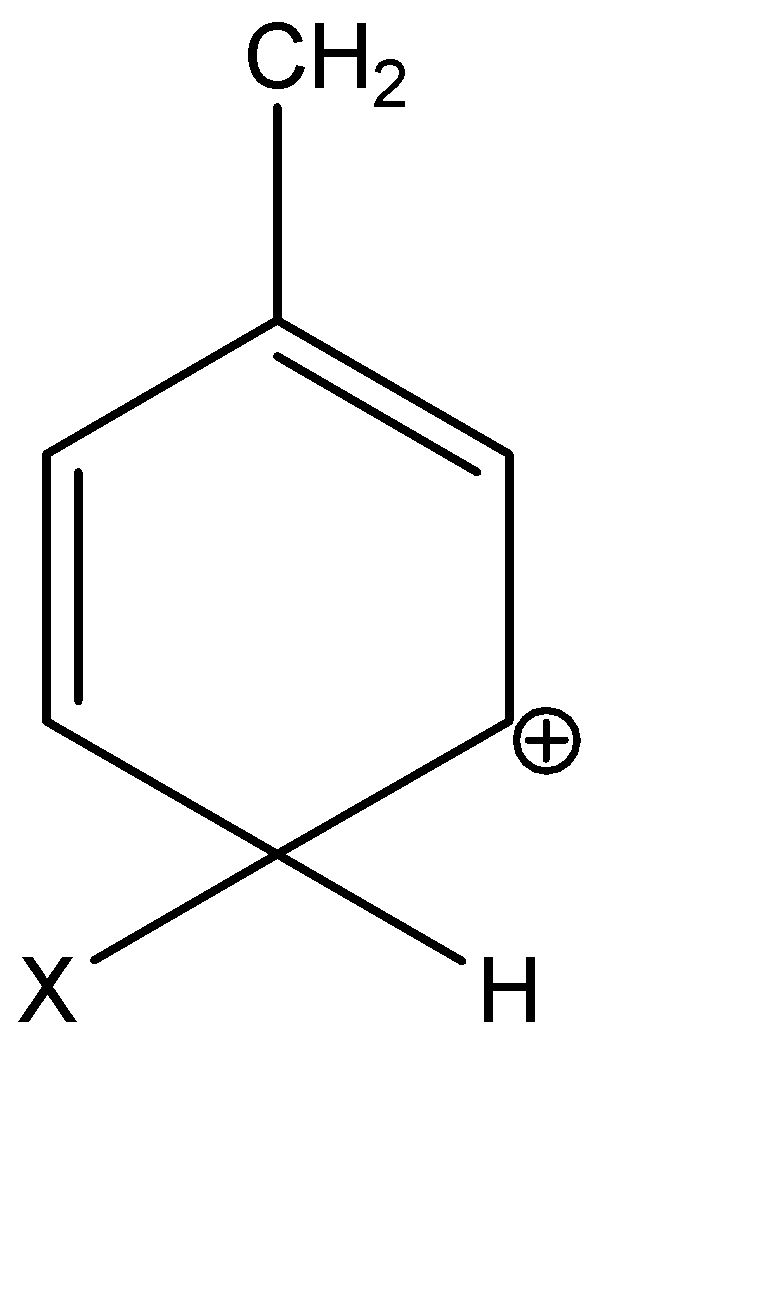
B) 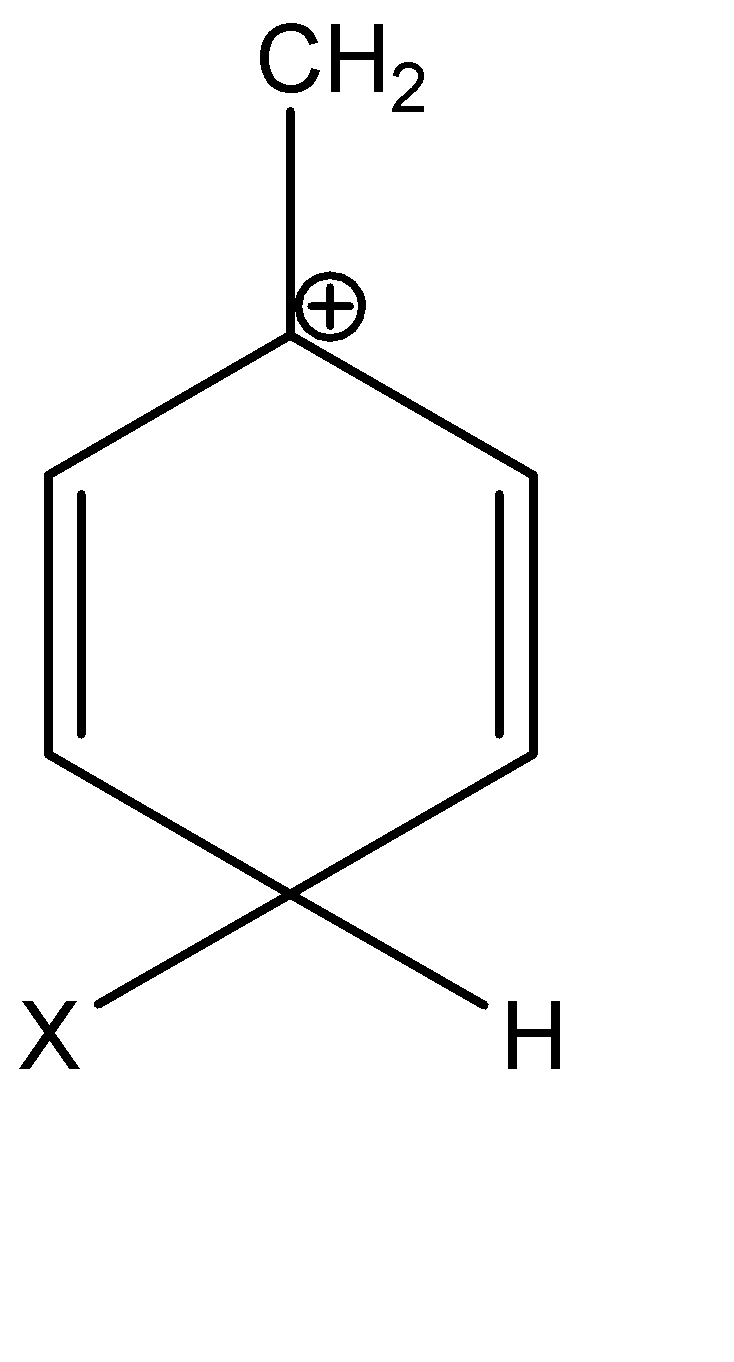
C) 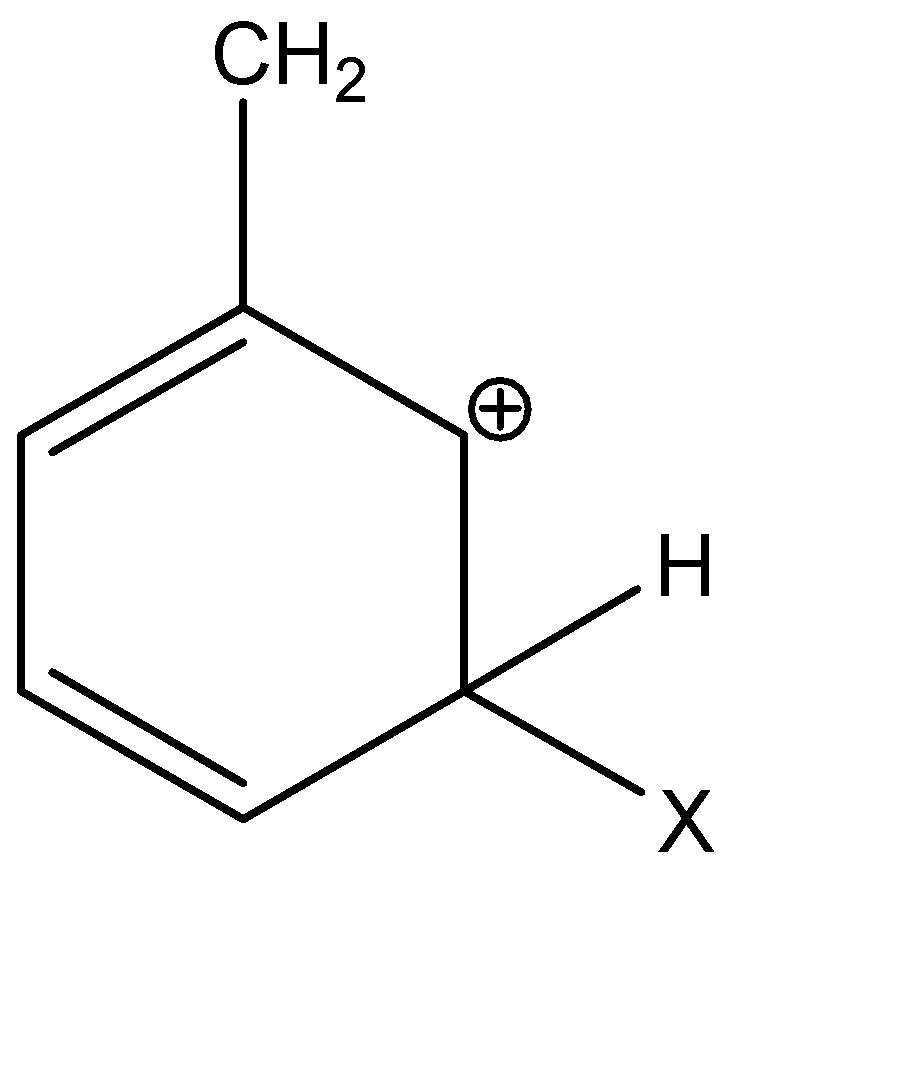
D) 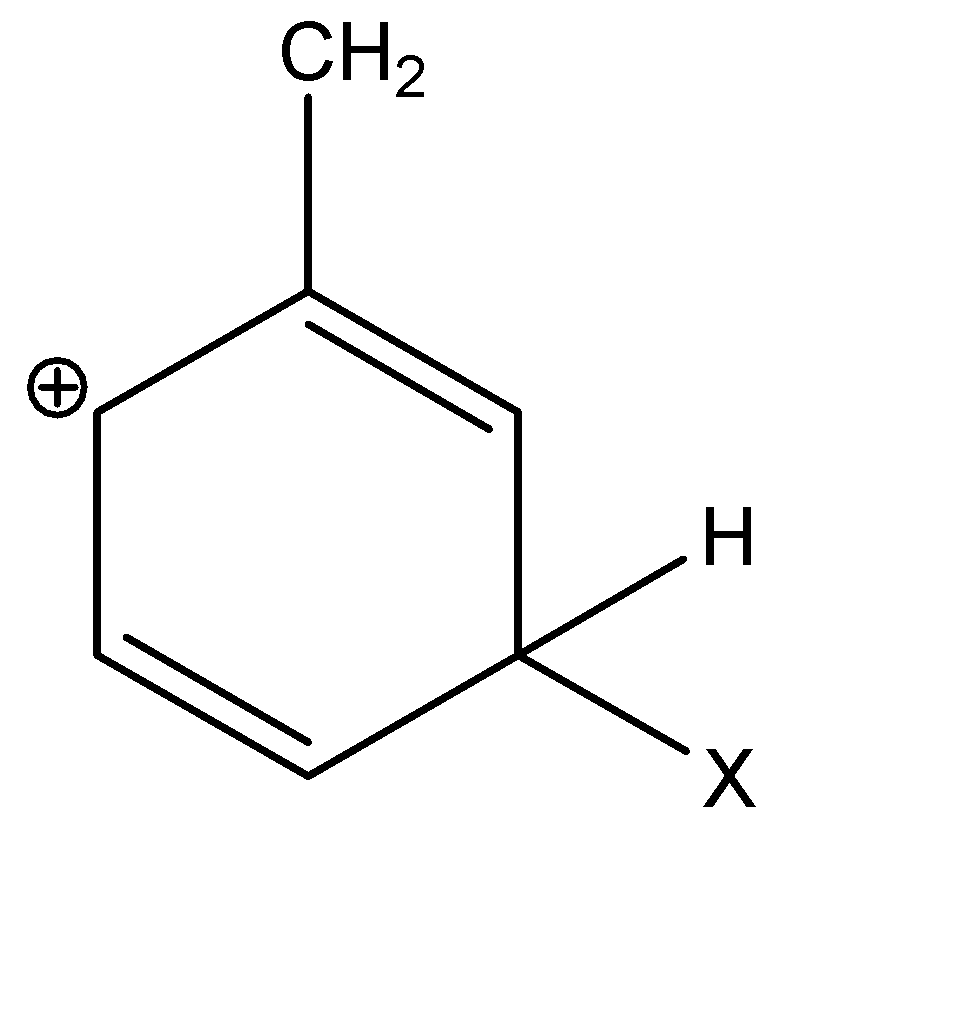
Solution
The stability of the carbocation depends on several factors, but here we will use the factor considering the effect of electron donating groups on the stability of carbocation. We have been given a methylene group.
Complete answer:
A carbocation is the species with a positive charge on it, which means that it is electron deficient. The species will be stabilized only if it is attached to an electron donating group, which will supply electrons to the deficient species. A positively charged species (carbocation) is electron poor, and thus anything which will donate electron density to the centre will help stabilize it. Electron withdrawing groups on the other hand destabilizes it.
Groups like ethyl, methyl, methylene, etc are weak electron donating groups, and can stabilize a carbocation. In carbocations, the ones that are highly substituted are stable. Example, tert- butyl carbocation is more stable than an isopropyl carbocation. Primary carbocations are very unstable. Hence, if the species forms 3∘ carbocation it will be the most stable.
Alkyl groups are electron donating groups and carbocation stabilizing, because the electrons on the neighbouring carbons are attracted towards the positive charge, slightly reducing the electron poverty. In the given options, A, C and D are secondary carbocations.
Option B is a tertiary carbocation; thus, it is the most stable. Option B is the correct answer.
Note:
Electron withdrawing groups highly destabilize the carbocation. Example, Carbonyl groups are electron withdrawing due to the polarity of the C=O bond. If the electron withdrawing groups are present, then the species which has the positive charge at the farthest sigma bond distance from the group, will be the most stable.
