Question
Question: Which of the following cannot be the product of this reaction? Dehydration of: 
A) 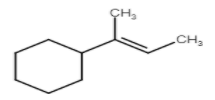
B) 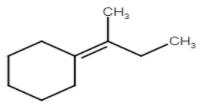
C) 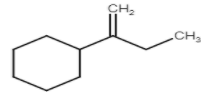
D) 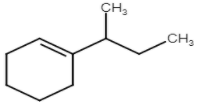
Solution
Alcohols when reacted with protonic acids lose water molecules to form alkenes. It’s an elimination reaction and it depends upon carbocation stability and degree of alcohol. When H+ ion from acid attacks a –OH group a water molecule is removed and a carbocation is formed then by elimination reaction an alkene is formed.
Complete step by step answer:
Dehydration of alcohol is defined as the removal of a hydrogen molecule from the alcohol molecule upon reaction with some protonic acid.
Dehydration of alcohol can be observed in three steps:
- Formation of protonated alcohol.
- Formation of carbocation.
- Formation of alkene.
Let us discuss the mechanism involved in the reaction.
1. Formation of protonated alcohol: When a protonic acid attacks on the alcohol. Oxygen is electron rich due to lone pairs so it acts as Lewis base. Protonation of oxygen makes it a good leaving group. This is a reversible process so it takes place very quickly.
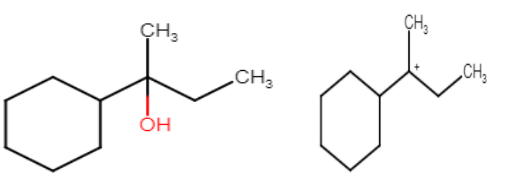
2.Formation of carbocation: This is the most important step in this reaction and it is the slowest process so it is the rate determining step for this reaction. Here rearrangement of carbocation takes place for maximum stability.
Stability of carbocation is 3∘>2∘>1∘. But in this question we see that carbocation formed after protonation is already 3 degrees which is more stable.
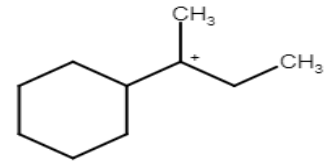
3. Formation of Alkene: Here the elimination of hydrogen takes place. The carbon adjacent to carbocation leaves the hydrogen and form a double bond
Here are three carbons adjacent to carbocation so there are three possible products but their stability is different due to hyper conjugation.
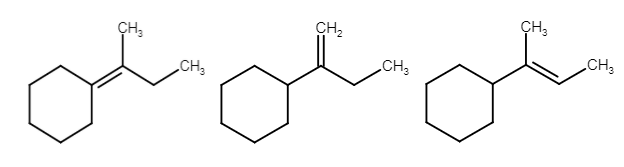
So these are the products which form on dehydration of given alcohol.
Hence the D option does not form.
Hence, the correct option is D.
Note: Remember to solve this type of question the most important thing is rearrangement of carbocation so always try to make carbocation most stable that is 3 degrees. Also when a major product is asked, always answer by seeing stability of alkene using hyperconjugation.
