Question
Question: Which of the following cannot be a base? A.
B.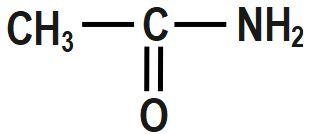
C.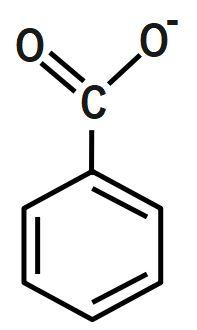
D.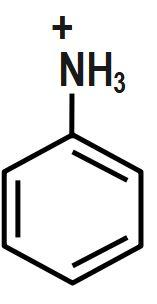
Solution
We know that we know that base is a substance, which could neutralize acid. When an acid and base react together, the product formed will be salt and water. We can classify base into two types: Strong base and weak base.
Complete answer:
Bases that are water-soluble are called alkali. Both alkali and bases are made up of metal oxides, metal hydroxides, metal carbonates. A base is a substance, which neutralizes an acid. Base has the ability to accept a proton from an acid. Some of the bases such as metal hydroxides and metal oxide form neutral products on reaction with acids. Base is insoluble in water. Salts and water are produced when the base reacts with acids. They have bitter taste and are slippery. There are two types of bases: strong and weak bases.
Bases accept protons and form stable conjugate acids. By observation, we can say that in Option D, there is already a positive charge over the electronegative N-atom. Hence, it will be reluctant to accept any more protons. Thus, it cannot act as a base.
Therefore, the correct answer is option D.
Note:
Remember that the alkalis are bases (or) hydroxide that belongs to the s-block element that is alkali metal and alkali earth metal. Alkali has the ability to accept a proton, and gives out hydroxide ion (OH−) . Some of the applications of alkali are, Sodium hydroxide is used in the manufacture of paper, detergents and soaps.
