Question
Question: Which of the following can undergo aldol condensation? This question has multiple answers. A) Ac...
Which of the following can undergo aldol condensation?
This question has multiple answers.
A) Acetaldehyde
B) Propanaldehyde
C) Benzaldehyde
D) Tri deutro acetaldehyde
Solution
We must have to know that an aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone. This reaction provides a good way to form carbon–carbon bonds.
Complete answer:
As we know, the basic requirement for aldol condensation is the presence of alpha hydrogen. So we will look at all the options one by one and then select the answer. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals.
Option A) this option is correct as acetaldehyde has three alpha hydrogen as confirmed by its structure, thus it will give aldol condensation.
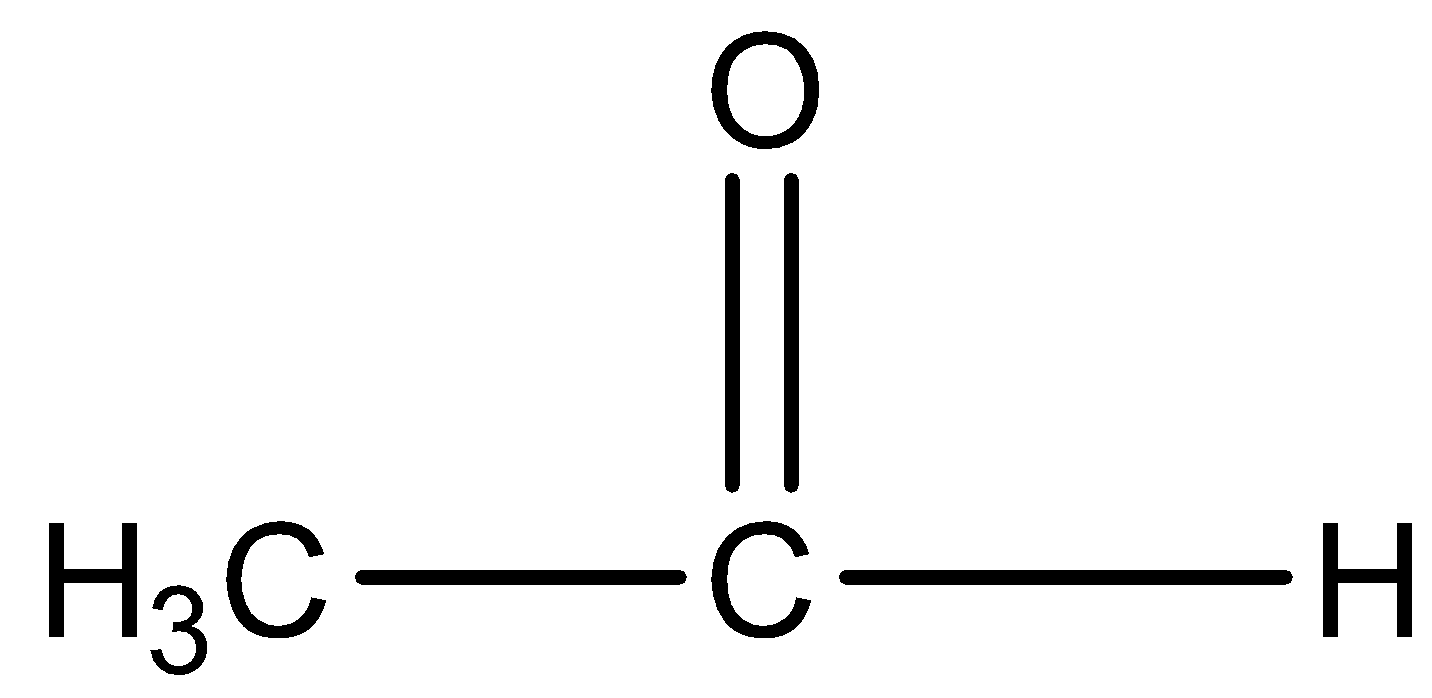
Option B) this option is also correct as propanaldehyde has two alpha hydrogens as confirmed by its structure, thus it will give aldol condensation.
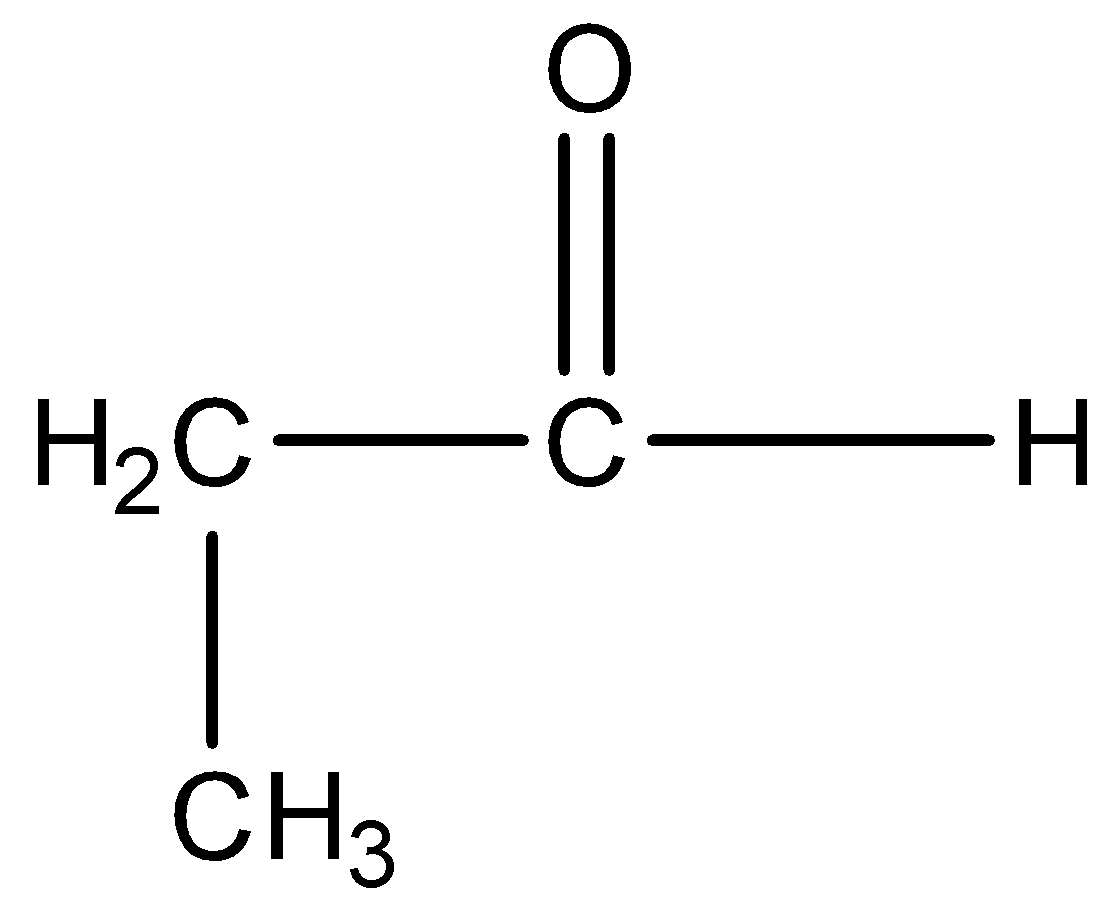
Option C) this option is incorrect as benzaldehyde has no alpha hydrogens, thus it will not give aldol condensation.
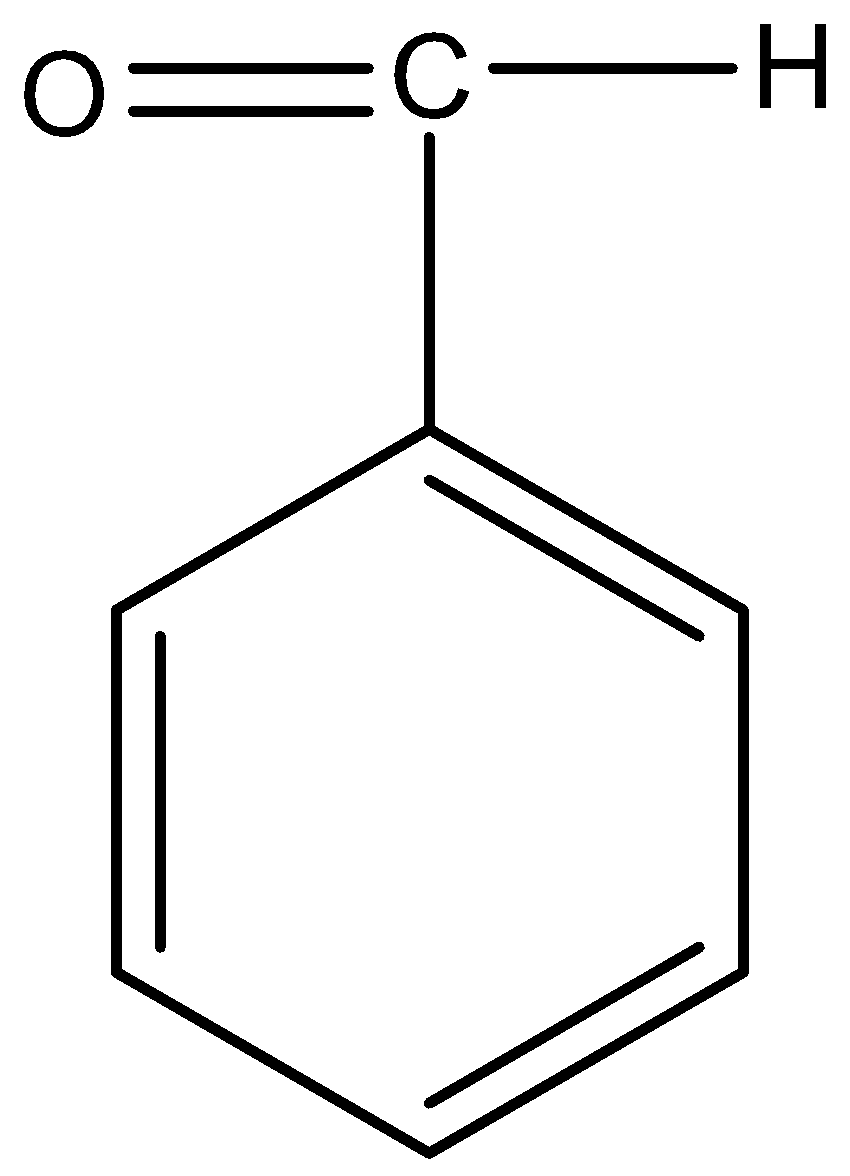
Option D) this option is correct as trideutroaldehyde has three deuterium atoms thus it will give aldol condensation.
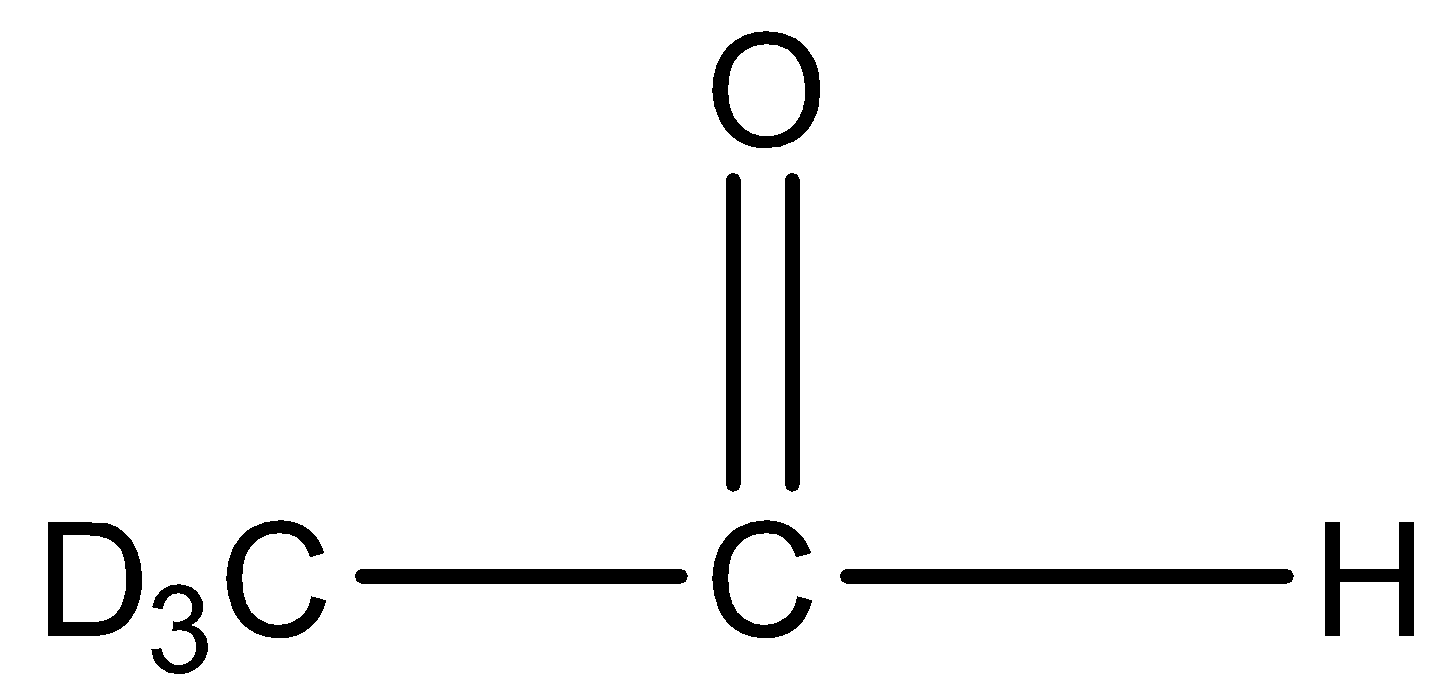
Option A B and D is the correct answer.
Note:
The name aldol condensation is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, the aldol reaction is not formally a condensation reaction because it does not involve the loss of a small molecule.
