Question
Question: Which of the following can show geometrical isomerism? A.
B.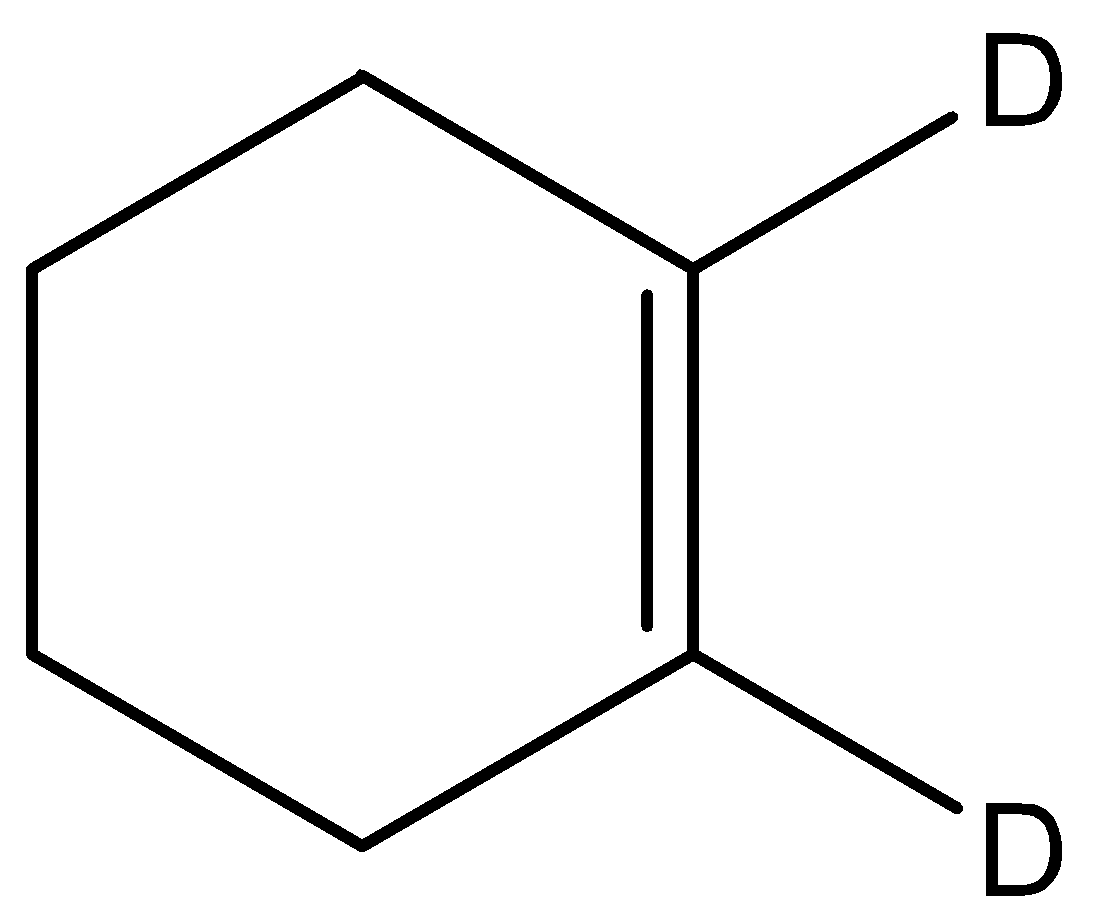
C.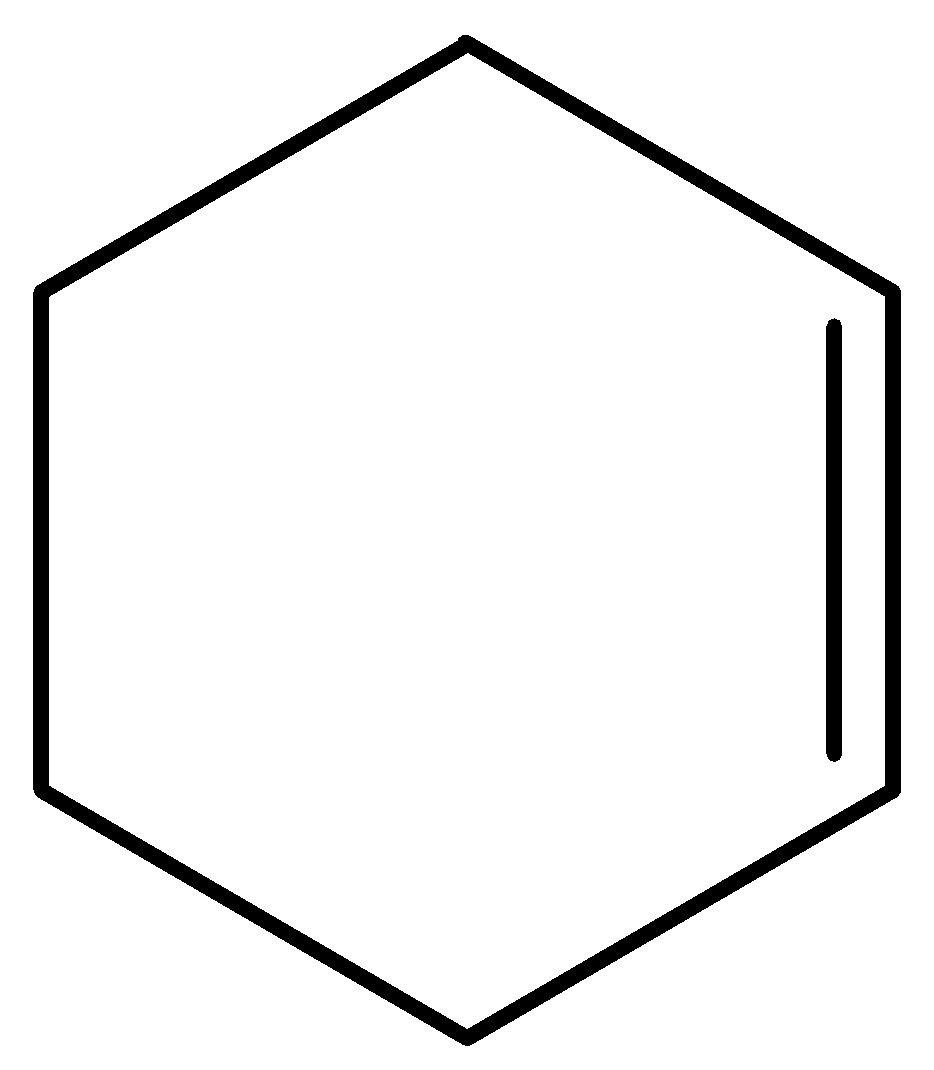
D. All of the above
Solution
We first need to understand the concept of isomerism. Isomerism in chemistry, is the phenomenon where molecules have the same molecular formula,i.e. same number of atoms but their arrangements in space is different and these molecules are known as isomers.There are two primary types of isomerism- Structural Isomerism and Stereoisomerism. In structural isomerism, the atoms and functional groups of the molecule (isomer) are linked in different ways.
Complete step by step answer:
Geometrical isomerism is a type of stereoisomerism whose characteristics are given below:
This is also known as cis-trans isomerism.
They have different spatial arrangements in the three-dimensional space.
Geometric Isomerism are commonly observed in Carbon-Carbon double bonds.
Carbon-carbon double bonded compounds have restricted rotation.
Geometric isomers can occur where there is restricted rotation about a bond.
To know whether a molecule exhibits geometrical isomerism or not, the molecule must:
Restrict Rotation involving a carbon-carbon double bond.
There should be two different compounds on the left hand side and right-hand side of the double bond.
Let us look at each of the given molecules one by one.
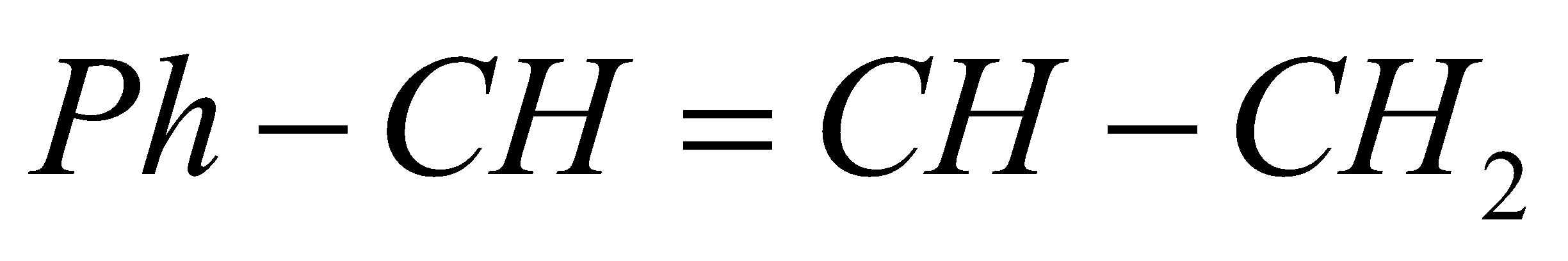
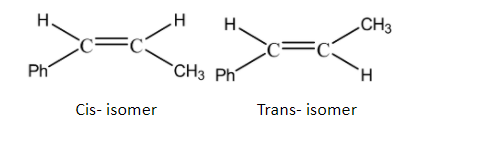
This molecule fulfils the criteria of exhibiting geometrical isomerism as it has a double bond and the groups on either side of the bond are different. Hence it can exhibit cis-trans isomerism as given below:
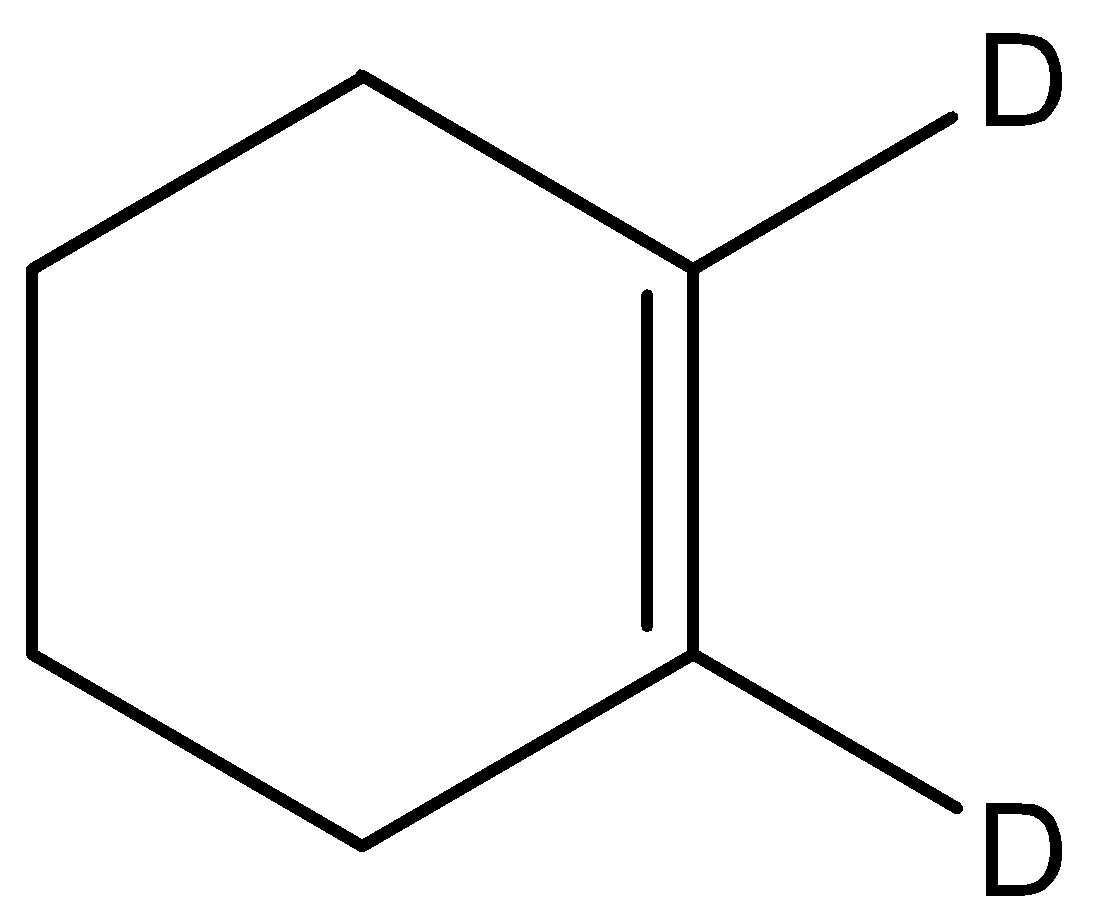
This molecule has a double bond but the groups on either side of the double bond are the same hence cannot exhibit geometrical isomerism.
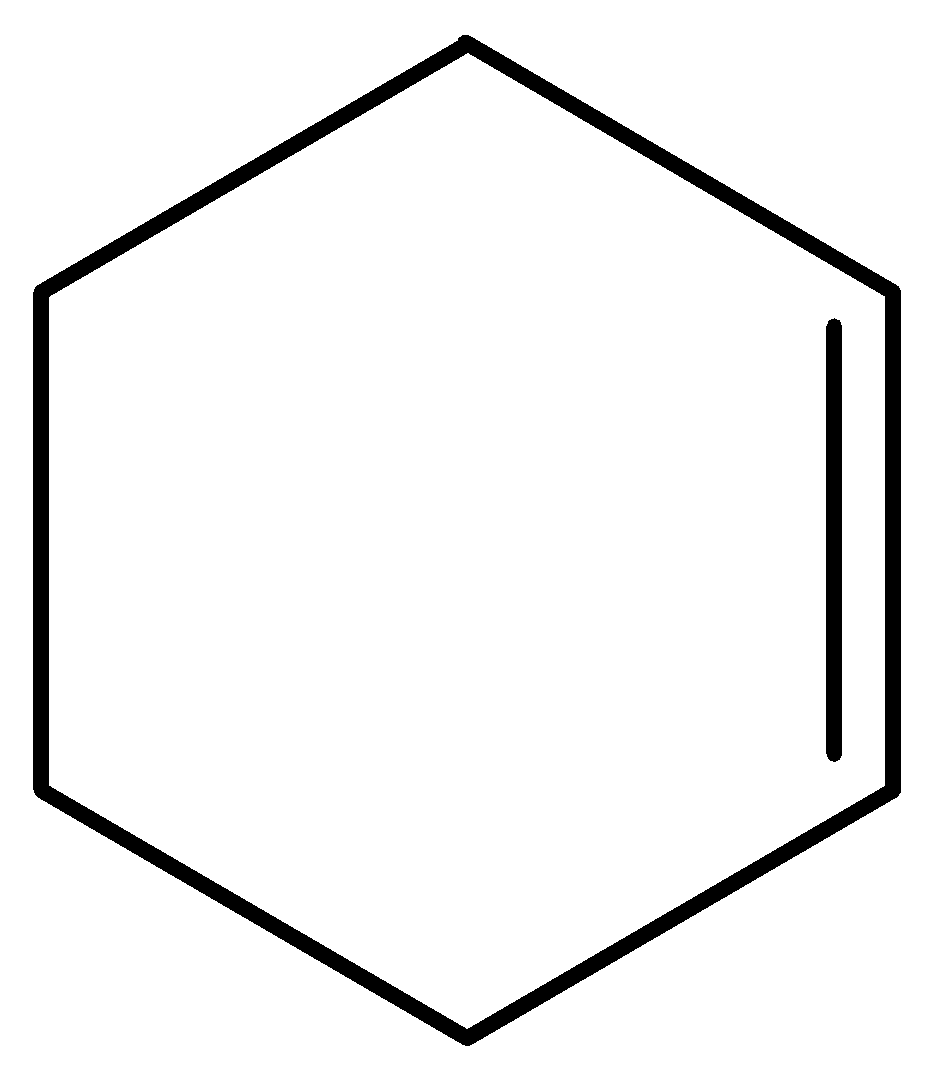
This molecule has no groups on either side of the double bond hence cannot show geometrical isomerism.
So, the correct answer is Option A.
Note: It must be noted that the most important criteria for an organic compound to exhibit geometrical isomerism is presence of a double bond with different groups present on either side of the bond. The atoms or functional groups attached to the double bonded carbons must be different. Hence it is obvious that alkanes do not exhibit geometrical isomerism.
