Question
Question: Which of the following can be used as the halide component for Friedel-Crafts reaction? A. Chloro...
Which of the following can be used as the halide component for Friedel-Crafts reaction?
A. Chloroethene
B. Isopropyl chloride
C. Chlorobenzene
D. Bromobenzene
Solution
Friedel-Crafts electrophilic aromatic substitution reaction. An alkyl halide is used as the reagent in Friedel-Crafts alkylation reaction where hydrogen atom of an aromatic ring is replaced by the alkyl group. In Friedel-Crafts alkylation reaction carbocation is formed as intermediate.
Complete step by step answer:
In Friedel-Crafts alkylation reaction the first step is the formation of a carbocation. Alkyl halide reacts with Lewis acid and gives electrophilic carbocation.
The general reaction of an alkyl halide with Lewis acid is as follows:
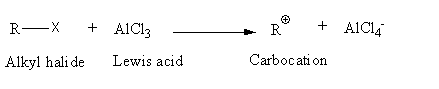
Draw the structures of all halide components given.
A) Chloroethene:
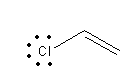
In chloroethene due to the presence of double bond lone pair on chlorine is delocalized and attains the double bond character. So chloroethene shows less reactivity with Lewis acid and hence cannot be used as a reagent for Friedel-Crafts alkylation reaction.
So, the option is (A) Chloroethene is an incorrect answer.
B) Isopropyl chloride:
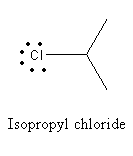
As there is no double bond, lone pairs on chlorine in Isopropyl chloride react with Lewis acid and give secondary carbocation.
So, the option is (B) Isopropyl chloride is the correct answer.
C) Chlorobenzene:
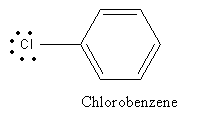
In the case of chlorobenzene lone pair on chlorine is delocalized in benzene ring and so show less reactivity with Lewis acid and hence cannot be used as a reagent for Friedel-Crafts alkylation reaction.
So, the option is (C) Chlorobenzene is an incorrect answer.
D) Bromobenzene
In the case of bromobenzene, the lone pair on bromine is delocalized in benzene ring and so shows less reactivity with Lewis acid and hence cannot be used as a reagent for Friedel-Crafts alkylation reaction.
So, the option is (D) Bromobenzene is an incorrect answer.
Thus, the correct option is (B) Isopropyl chloride is the correct answer.
Note: Reactivity of halogens decreases due to the delocalization of lone pairs of halogen across the double bond or an aromatic ring. So haloalkanes and phenyl halide cannot be used as a reagent in Friedel-Crafts alkylation reaction.
