Question
Question: Which of the following can be produced by wurtz reaction in good yield? (A)  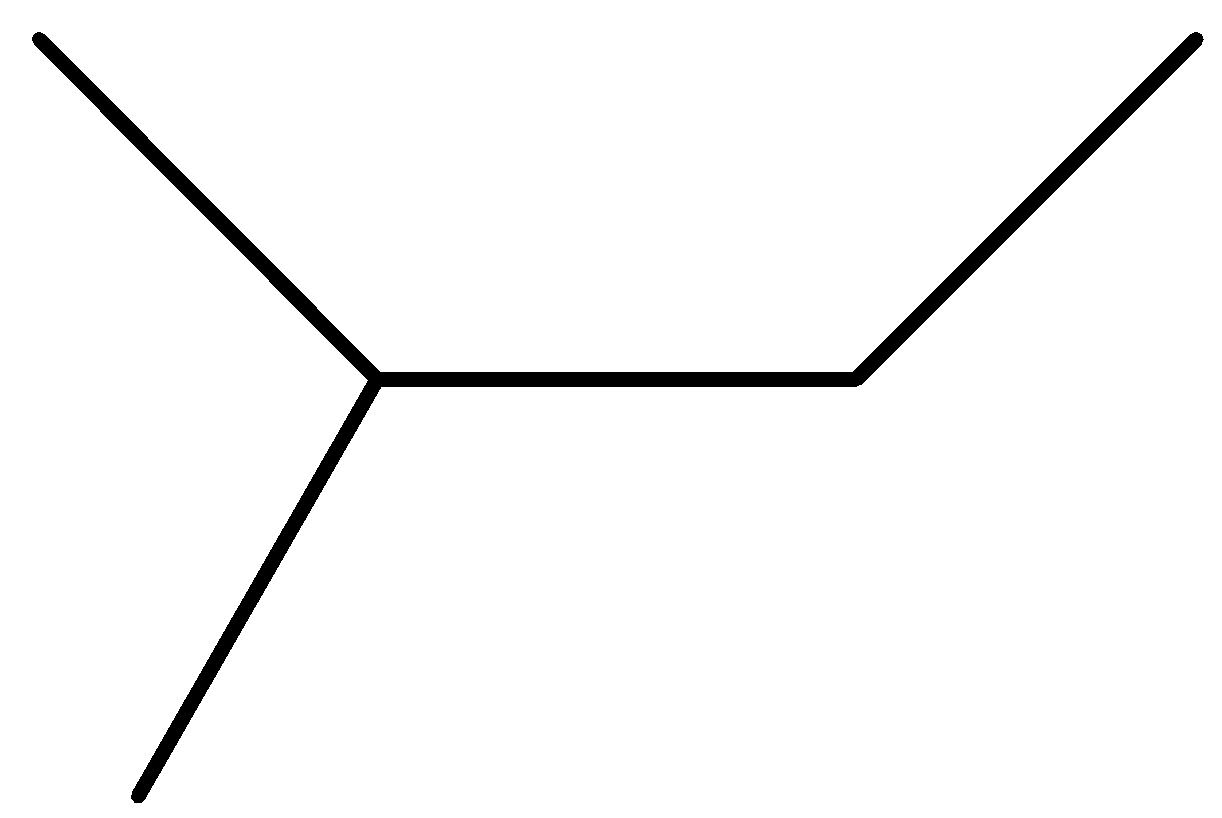
(B) 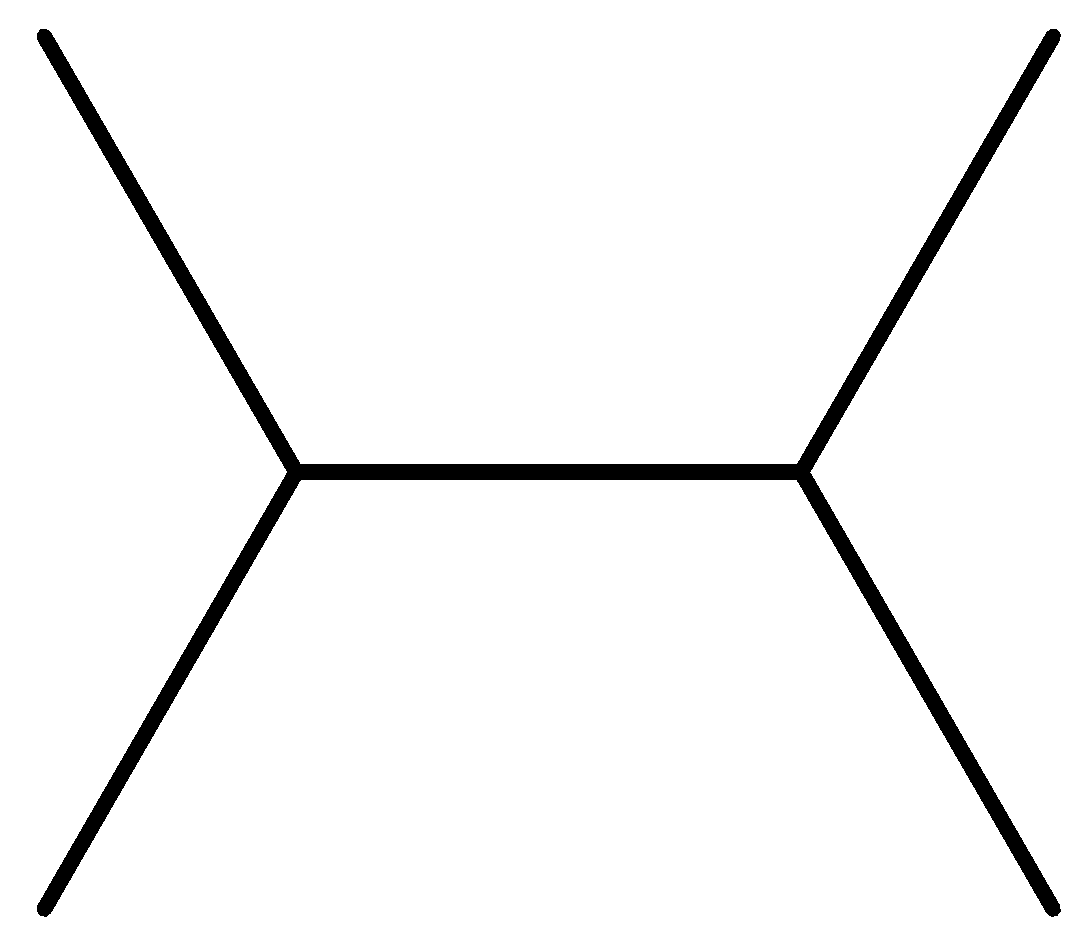
(C) 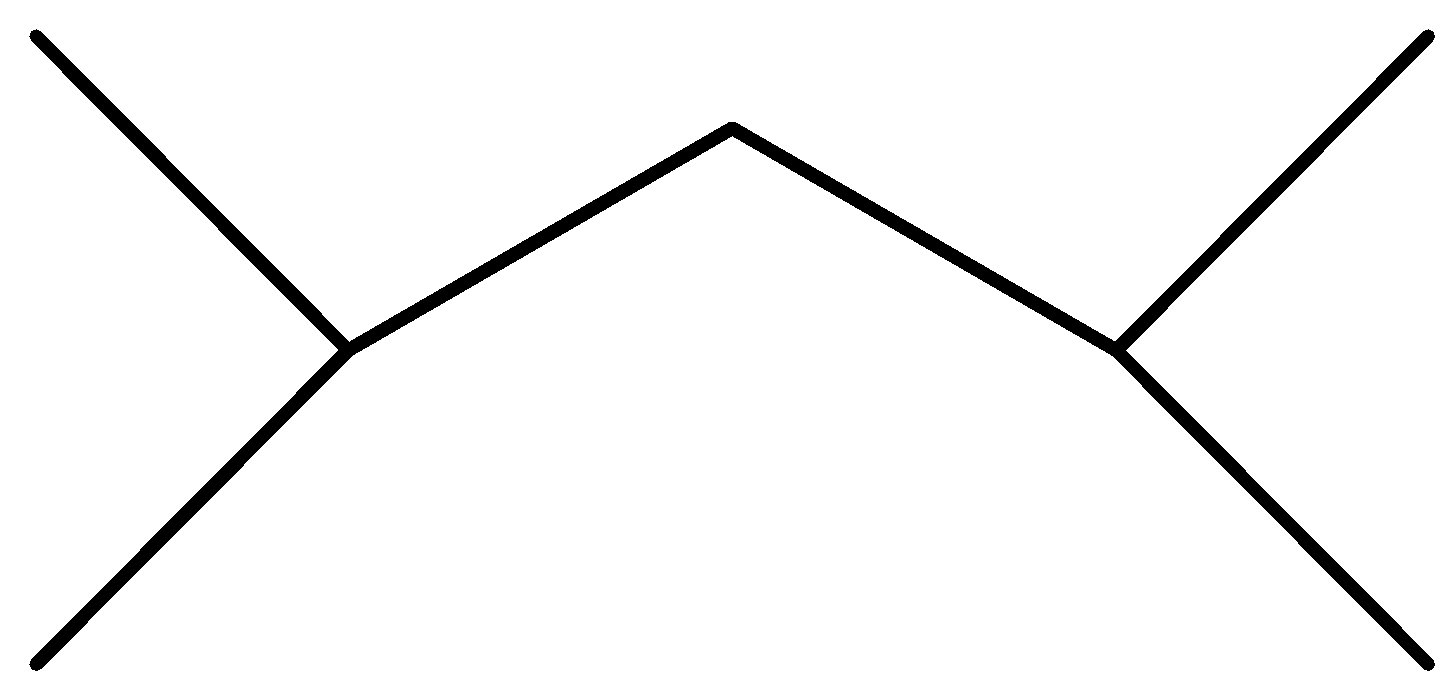
(D) 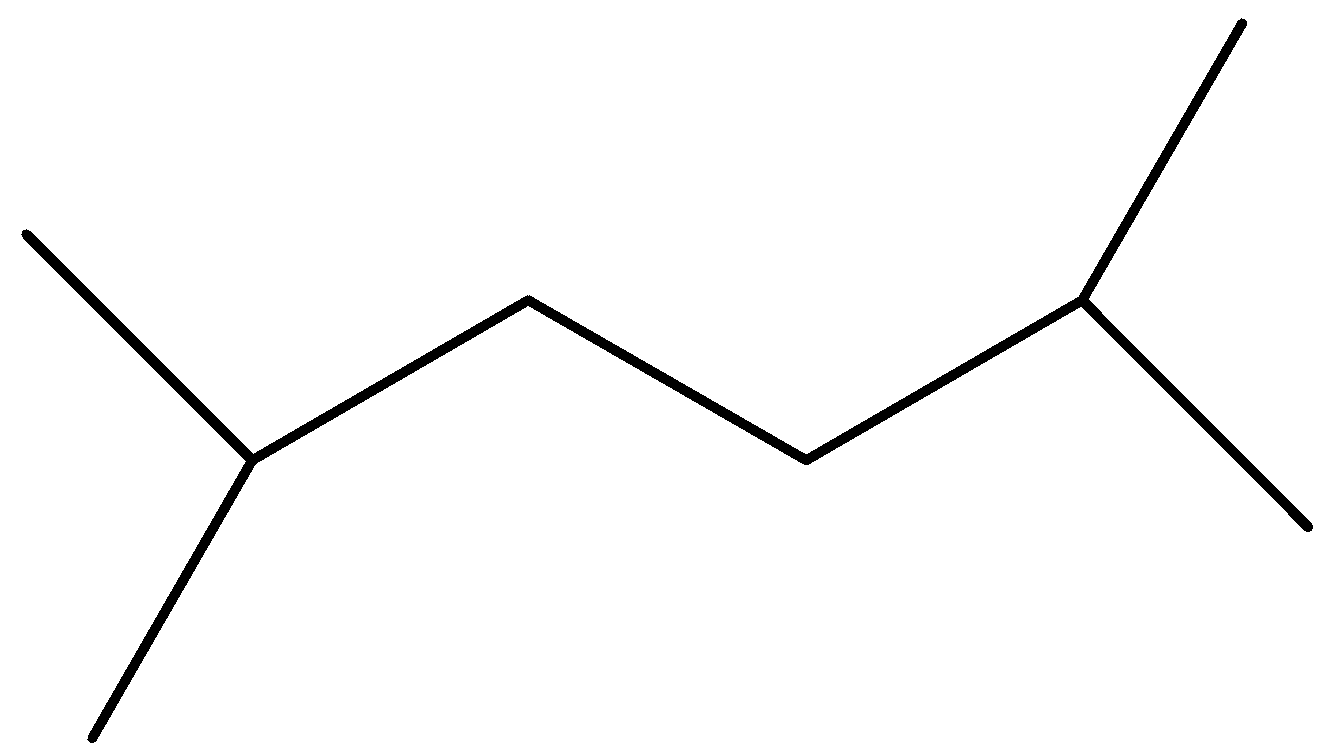
Solution
First we will learn about the mechanism and conditions for a wurtz reaction as to what kind of substrate it acts upon and then we will know what kind of substrate produces a high yield product according to the mechanism.
Complete step by step answer:
Wurtz reaction is a type of coupling reaction. A coupling reaction in which metal is used so it is a type of organometallic reaction. In this reaction two molecules of alkyl halide couple with each other in presence of sodium metal and dry ether to produce a higher alkane. The two molecules of alkyl halide used can be the same or can be different also. But a very good yield will be produced only when the reactants are same and symmetrical.
Mechanism of wurtz reaction is:
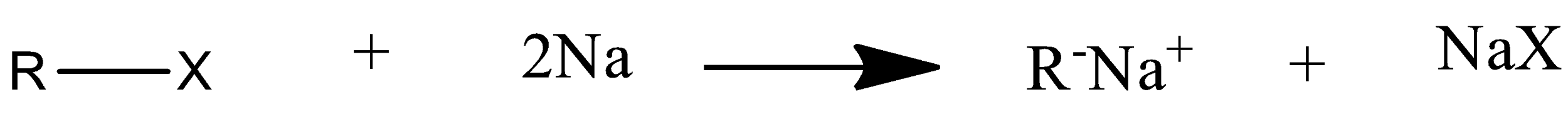
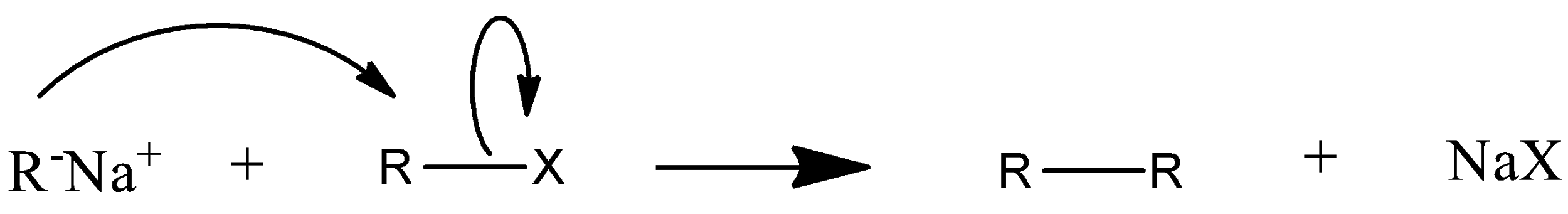
So this is how a higher alkane is formed from wurtz coupling.
Now for good yield the reactants must be symmetric and reactants must be the same.
Option A is not symmetrical so not the correct answer.
Option B is symmetrical as well as the reactant will be similar as 2 - chloropropane
Option C is not symmetrical and reactants will also not be the same. One will be 2 - chloropropane other will be 1 - chloro - 2 - methylpropane.
Option D is symmetrical but again the reactants are not similar.
So, the correct answer is Option B.
Additional Information:
Just like the wurtz reaction there is a wurtz fittig reaction also which is also a coupling reaction. In wurtz fittig the reactants are aryl halides with alkyl halides so that we can form aromatic products also.
Note: In wurtz coupling reaction it is mandatory to use only dry ether and not moist ether . The reason is that the moist ether contains water molecules and we all are aware that sodium reacts violently with water so the reaction cannot be done in moist condition.
