Question
Question: Which of the following can be isolated as the product of this reaction? 
A)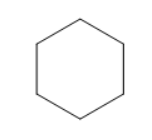
B)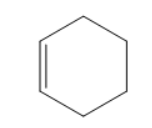
C)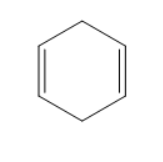
D)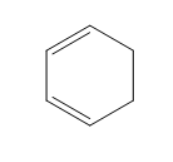
Solution
In order to solve this question we need to know what happens when hydrogen and nickel reacts with benzene in high temperature and high pressure. So, it is basically a hydrogenation reaction. These reactions devastate the electron delocalization in the first benzene ring on the grounds that those electrons are being utilized to shape bonds with the new hydrogen particles.
Complete step by step answer:
Hydrogenation of benzene:
These reactions devastate the electron delocalization in the first benzene ring on the grounds that those electrons are being utilized to shape bonds with the new hydrogen particles.
In spite of the fact that the responses are exothermic generally speaking as a result of the qualities of all the new carbon-hydrogen bonds being made, there is a high enactment barrier to the reaction.
The reactions are finished utilizing the very finely partitioned nickel catalyst that is utilized in hydrogenating alkenes and at comparative temperatures, yet the pressures utilized will in general be higher.

The catalytic hydrogenation of benzene has been done as a model response to build the hydrogenated cyclic mixes from aromatics. Impetus tests containing nickel on various backings were arranged and tried. It was discovered that α−Al2O3 upheld nickel demonstrated the best movement for benzene transformation response. Nickel metal region, its scattering, and nickel crystal size was resolved. The best action is gotten with 40% nickel focus (as oxide) and at the ideal nickel crystallite size of 196A∘ and ideal metal territory of 10.8m2/g.
So, upon hydrogenation of benzene we get cyclohexane as a major product as shown in the picture.
So, the correct answer is Option A.
Note: In spite of the fact that hydrogenation of benzene happens, it just happens under states of either high temperature or high pressure and within the sight of an exceptionally active catalyst. This is on the grounds that benzene is balanced out by the delocalization of electron thickness around its six carbons and is supposed to be aromatic. Because of its high initiation energy, the reaction won't occur at an impressive rate under ordinary conditions and that is the reason a high energy condition (high temperature) should be utilized for the reaction to really happen. In spite of the fact that the response is exothermic, it isn't close to as exothermic as it should be. This is true because of the way that benzene is aromatic.
