Question
Question: Which of the following calcium salt will give cyclopentanone on heating? A. calcium succinate B....
Which of the following calcium salt will give cyclopentanone on heating?
A. calcium succinate
B. calcium adipate
C. calcium glucarate
D. calcium oxalate
Solution
It is given that some calcium salt gets heated to produce cyclopentanone. So we have to find the reactant which is a calcium salt. Calcium salts of higher aromatic or aliphatic acids produce cyclopentanone, i.e. the reactant must have more than five carbon atoms.
Complete answer:
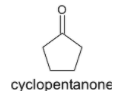
The chemical structure of cyclopentanone is given below:
Calcium salts of aliphatic or aromatic acids are obtained by reacting calcium hydroxide with corresponding acid.
Now let’s focus on each option.
A. When calcium succinate is heated, it produces cyclopropanone and calcium carbonate. Calcium succinate is prepared by reacting succinic acid with any calcium salt.
B. Calcium adipate is produced by reacting adipic acid with any calcium salt. When calcium adipate is heated, it produces cyclopentanone and calcium carbonate. The reaction is given below:
+CaCO3
C. When glucaric acid is treated with any calcium salt, it produces calcium glucarate. Also, when this calcium glucarate is heated, it produces a compound with chemical formula C5H8O5.
D. There is no particular product formed when calcium oxalate is heated.
Hence cyclopentanone is formed only when calcium adipate is heated. This reaction is called dry distillation. It is the process of heating a solid substance to give a gaseous substance. Aldehydes and ketones can be obtained using this method.
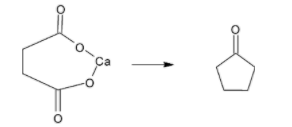
Hence, the correct option is B.
Note:
Symmetric ketones like acetone or diethyl ketone can be prepared by dry distillation of calcium salts of aliphatic and aromatic acids. Also, mixed ketones are obtained by reacting equal quantities of calcium salts of two different carboxylic acids.
