Question
Question: Which of the following bonds is strongest? A. 
B. 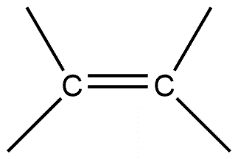
C. 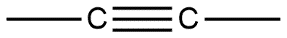
D. None of these
Solution
A molecule is composed by many atoms where we know that atom is the smallest structural unit which consist of a nucleus and nucleus contain positively charged ion known proton and neutral charge neutron inside it and negatively charged ions called electrons are revolving around the nucleus.
Complete answer:
These atoms are arranged in a molecule in some order or through some intermolecular forces of attraction or we can define it as a bonding present in the molecule. The bond may be covalent, ionic in nature.
Given compounds are discussed on the basis of bond present i.e. in compound A single bond is present which is also known by the name sigma bond. Whereas compound B double bond is present which contains one sigma bond and one pi bond, compound C contains a triple bond containing one sigma bond and two pi bonds in it.
Sigma bond is marked to be the strongest bond but if we compare these three molecules then we can see that compound A has only one sigma bond, B has one sigma and one pi bond whereas in compound C we have one sigma as well as 2 pi bonds. So this will be the strongest from all.
Hence option C is the correct answer.
Note:
The covalent bond is also termed as nonpolar because the difference in electronegativity is mostly negligible. This type of bond is also formed when atoms that share a polar bond arrange themselves in such a manner that electric charges between them tend to cancel each other.
