Question
Question: Which of the following are the properties of an electron? 1\. Electron is a constituent of cathode...
Which of the following are the properties of an electron?
1. Electron is a constituent of cathode ray.
2. Electron is a negatively charged particle.
3. The mass of the electron is equal to the mass of the proton.
4. Electrons are deflected by the electric field but not by the magnetic field.
Select the correct answer using the code given below.
A. 1 and 2 only
B. 1,2 and 3
C. 3 and 4
D. 1 and 4
Solution
Here we are given four points and we will analyse the property of electrons alongside with the points given. If the property of electrons turns out to be true that option is correct. First, we need to determine the charge and mass of an electron, then we need to understand how a charge particle behaves in an electric and magnetic field.
Complete answer:
Cathode rays are moving rays which are generated in a vacuum from a highly charged cathode and these rays move towards anode. Since cathode is negatively charged and anode is positively charged, we can conclude that the rays are electrons. So, point 1 is correct.
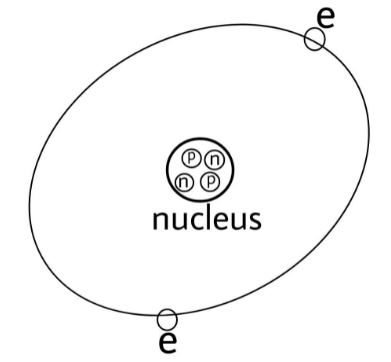
In the above diagram ‘p’ is for protons, ‘n’ is for neutrons and ‘e’ is for electrons.We all know that an atom consists of three fundamental particles: the proton, the neutron and the electron. Proton being positive and neutron being neutral acquires the nucleus of the atom and electrons being negative revolve around the nucleus.So, point 2 is correct.
We know from our elementary classes that proton weighs 1.6726×10−27kg and electron weighs $$9.1094×10−31kg . Thus, protons are 1837 times heavier than electrons.So, point 3 is incorrect.
Anything that has charge will be affected by the electric field. Since an electron has a negative charge, it is affected by the electric field.Anything that has charge and velocity will be affected by the magnetic field. Since an electron has both of these it will be affected by the magnetic field.So, point 4 is incorrect.
Therefore, the correct answer is option A.
Note: The same above mentioned stuff can be asked in case of proton, here protons will have positive charge will be present in anode rays which are generated from anode and move backwards which is in opposite direction if compared to cathode rays. And if it was the case of a neutron, we wouldn’t have observed any kind of rays emerging as it is neutral neither would it have been affected by any electric or magnetic field. But it has almost the same mass as that of a proton.
