Question
Question: Which of the following are peroxoacids of sulphur? A.\[{{\text{H}}_2}{\text{S}}{{\text{O}}_5}\] an...
Which of the following are peroxoacids of sulphur?
A.H2SO5 and H2S2O8
B.H2SO5 and H2S2O7.
C.H2S2O7 and H2S2O8
D.H2S2O6 and H2SO5
Solution
Hint : A large number of oxyacids-are known in the case of sulphur either in free state or in the form of salts or both. Acids which contain O−O linkage are known as peroxoacids of sulphur. From the structures of the given compounds, it can be found that they contain O−O linkage.
Step-by-step answer:
Sulphur produces a number of oxyacids. The main oxyacids series of sulphur are sulphurous acid series, sulphuric acid series, thionic acid series and peroxyacid series.
Peroxoacids of sulphur contain peroxy linkage that is O−O linkage. This can be determined from the structures of the given compounds.
Lewis structure can be made by using the total number of valence electrons needed by all atoms to achieve noble gas configuration.
In H2SO5, there are two hydrogen atoms, one sulphur atom and five oxygen atoms. Thus the total number of valence electrons (V.E) can be calculated as follows:
Totalvalenceelectrons=(2×V.EofH)+(1×V.EofS)+(5×V.EofO)
Since we know that the atomic number of hydrogen is 1 and its electronic configuration is 1. This shows that there is 1 electron in the outermost shell (valence shell). Thus the valence number of hydrogen is 1. The atomic number of sulphur is 16 and its electronic configuration is 2, 8, 6. This shows that there are 6 electrons in the outermost shell. Thus the valence number of sulphur is 6. The atomic number of oxygen is 8 and its electronic configuration is 2, 6. This shows that there are 6 electrons in the outermost shell. Thus the valence number of oxygen is 6.
Hence the total number of valence electrons in H2SO5 can be calculated as:
{\text{Total}}\;{\text{valence}}\;{\text{electrons}} = \left( {2 \times 1} \right) + \left( {1 \times 6} \right) + \left( {5 \times 6} \right) \cr = 2 + 6 + 30 \cr = 38 \cr} $$ This means that there are a total 38 valence electrons needed by all atoms to acquire noble gas configuration. On the basis of these valence electrons, Lewis structure can be made. When each atom acquires complete octet, Lewis structure is ready as follows: 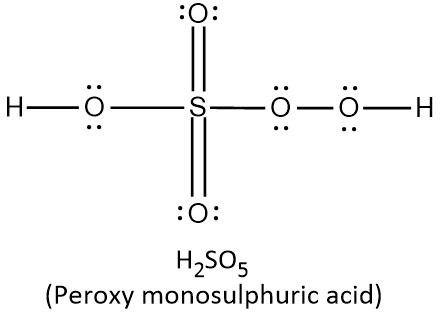 It can be observed from the above structure that there is a peroxy linkage $$\left( {{\text{O}} - {\text{O}}} \right)$$. Thus $${{\text{H}}_2}{\text{S}}{{\text{O}}_5}$$ is a peroxyacid of sulphur. In $${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{8}}}$$ , there are two hydrogen atoms, two sulphur atoms and eight oxygen atoms. Thus the total number of valence electrons in $${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{8}}}$$ can be calculated as: $$\displaylines{ {\text{Total}}\;{\text{valence}}\;{\text{electrons}} = \left( {2 \times 1} \right) + \left( {2 \times 6} \right) + \left( {8 \times 6} \right) \cr = 2 + 12 + 48 \cr = 62 \cr} $$ This means that there are a total 62 valence electrons needed by all atoms to acquire noble gas configuration. On the basis of these valence electrons, Lewis structure of $${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{8}}}$$ is ready as follows: 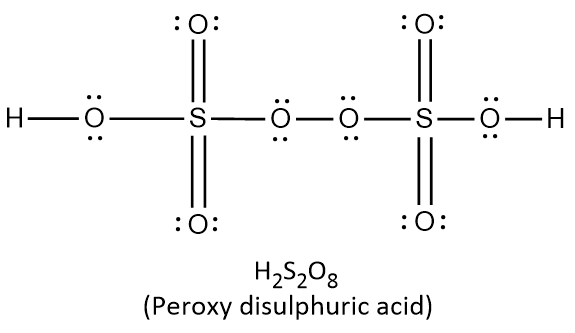 It can be observed from the above structure that there is a peroxy linkage $$\left( {{\text{O}} - {\text{O}}} \right)$$. Thus $${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_8}$$ is a peroxyacid of sulphur. In $${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{7}}}$$ , there are two hydrogen atoms, two sulphur atoms and seven oxygen atoms. Thus the total number of valence electrons in $${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{7}}}$$ can be calculated as: $$\displaylines{ {\text{Total}}\;{\text{valence}}\;{\text{electrons}} = \left( {2 \times 1} \right) + \left( {2 \times 6} \right) + \left( {7 \times 6} \right) \cr = 2 + 12 + 42 \cr = 56 \cr} $$ This means that there are a total 56 valence electrons needed by all atoms to acquire noble gas configuration. On the basis of these valence electrons, Lewis structure of $${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{7}}}$$ is ready as follows: 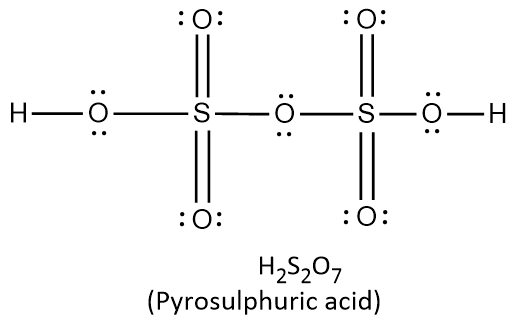 It can be observed from the above structure that there is no peroxy linkage. Thus $${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_8}$$ is not a peroxyacid of sulphur. In $${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{6}}}$$ , there are two hydrogen atoms, two sulphur atoms and six oxygen atoms. Thus the total number of valence electrons in $${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{6}}}$$ can be calculated as: $$\displaylines{ {\text{Total}}\;{\text{valence}}\;{\text{electrons}} = \left( {2 \times 1} \right) + \left( {2 \times 6} \right) + \left( {6 \times 6} \right) \cr = 2 + 12 + 36 \cr = 50 \cr} $$ This means that there are a total 50 valence electrons needed by all atoms to acquire noble gas configuration. On the basis of these valence electrons, Lewis structure of $${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{6}}}$$ is ready as follows: 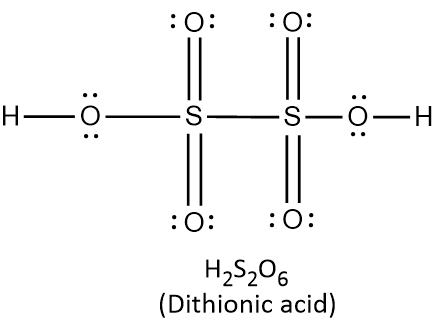 It can be observed from the above structure that there is no peroxy linkage. Thus $${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_6}$$ is not a peroxyacid of sulphur. Therefore, the correct option is A. Note: Remember that the oxyacids of sulphur which contain $${\text{O}} - {\text{O}}$$ linkage are peroxoacids of sulphur. The oxyacids which contain $${\text{S}} - {\text{S}}$$ linkage belong to thionic acid series. The oxyacids which contain $${\text{S}} - {\text{O}} - {\text{S}}$$ linkage belong to sulphuric acid series.