Question
Question: Which of the following are conjugate acid-base pairs? A. \(HC{{O}_{3}}^{-},C{{O}_{3}}^{2-}\) B. ...
Which of the following are conjugate acid-base pairs?
A. HCO3−,CO32−
B. C6H5NH3+,C6H5NH2
C. H2C2O4,HC2O4−
D. OH−,H+
Solution
Generally all acids are sour in taste and turn blue litmus paper into red while bases are bitter in taste and turn red litmus paper into blue. The pH value of acids are greater than 7 and for bases it is less than 7 and if the value of pH is exactly 7 then it indicates the given solution is neutral in nature.
Complete answer:
Conjugate acid are those acids which contain one more H atom and one more positive charge than the base from which it is formed and other the other hand conjugate base are those bases which contain one less H atom and one more negative charge than the acid from which it is formed.
Whereas conjugate pair is made up by one conjugate acid and one conjugate base that’s why this is known by the name acid-base conjugate pair and this can be shown by the following image:
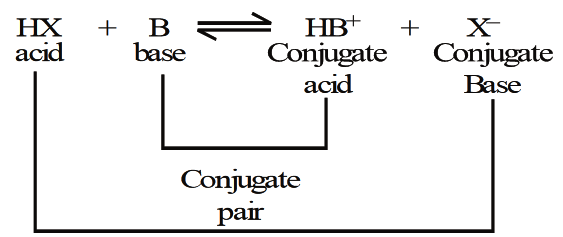
Here HX is acid which when react with base form the conjugate acid which have positive charge on it and reaction also contain an anion i.e. negative charge atom. In this way when acid is connected with conjugate base or base is connected with conjugate acid is known as conjugate acid-base pair.
In the given examples only OH−,H+ are not forming conjugate acid or base all other will.
Thus option A, B and C are correct.
Note:
An acid is defined as those substances which produce H+ in a solution whereas base produces OH− ions. Acids acts as a proton donor and electron pair acceptors while bases acts as a proton acceptor and electron pair donor.
