Question
Question: Which of the following are aromatic?...
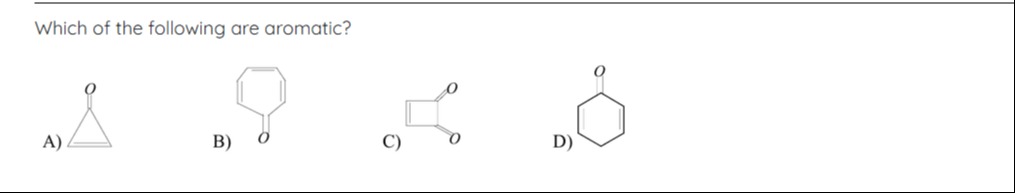
Which of the following are aromatic?
A
A) Cyclopropenone
B
B) Tropone
C
C) Squaric acid
D
D) Cyclohexenone
Answer
A, B, C
Explanation
Solution
To determine aromaticity, we check for the following criteria: 1. Cyclic, 2. Planar, 3. Continuous ring of delocalized pi electrons, 4. Huckel's Rule (4n+2 pi electrons).
- A) Cyclopropenone: Cyclic, planar, 2 π electrons (from C=C). Fits 4n+2 (n=0). Aromatic.
- B) Tropone: Cyclic, planar, 6 π electrons (from 3 C=C bonds). Fits 4n+2 (n=1). Aromatic.
- C) Squaric acid: Cyclic, planar, 6 π electrons (from 1 C=C and 2 C=O). Fits 4n+2 (n=1). Aromatic.
- D) Cyclohexenone: Cyclic, not fully planar, 4 π electrons (from 1 C=C and 1 C=O). Fits 4n (n=1). Non-aromatic.
