Question
Question: Which of the following annulenes are aromatic? A.[8]-Annulene B.[10]-Annulene C.[12]-Annulene ...
Which of the following annulenes are aromatic?
A.[8]-Annulene
B.[10]-Annulene
C.[12]-Annulene
D.None
Solution
If a molecule is aromatic, then it should fulfil these conditions: 1. It should be cyclic, planar and has continuous delocalization of π electrons (electrons in p orbitals) and the delocalized π-electron cloud must contain a total of (4n+2)π electrons (Huckel’s rule), where n is a whole number (i.e., n =0, 1, 2, 3 and so on).
Complete step by step answer:
Annulenes are the family of completely conjugated, monocyclic hydrocarbons containing alternating ring double bonds, such as benzene, but of different sizes. They have a general formula of CnHn , where ‘n’ is an even number or CnHn+1 , where n is an odd number.
The annulenes are named as [n]-annulene where n is an even number and it represents the number of Carbon atoms in the ring.
For example: Benzene is [6]-Annulene, cyclobutadiene is [4]-Annulene, cyclooctatetraene is [8]-Annulene.
Annulenes can be aromatic, anti-aromatic or non-aromatic. The behaviour of the annulenes can be explained on the basis of Huckel’s rule ( (4n+2)π electrons).
[8]-Annulene is also known as 1,3,5,7-cyclooctatetraene. It has a planar cyclic conjugated system which has 4nπ electrons, where n=2 . To overcome the strain molecule assumes a non-planar, tub-shaped geometry. So, it is non-aromatic
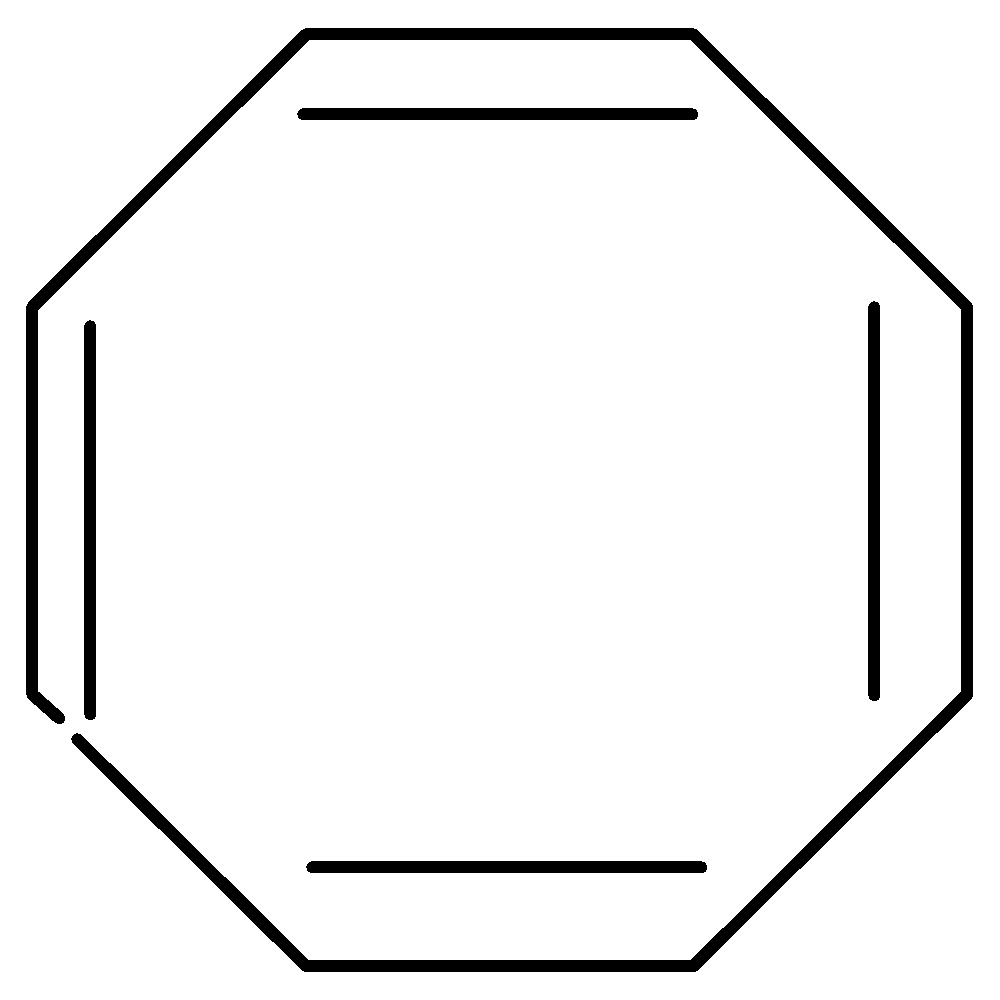
Figure: [8]-Annulene
[10]-Annulene is not particularly stable due to geometric factors. It possesses 10π electrons so it gives an indication of aromaticity as per the (4n+2)π electrons requirement for Huckel’s rule but it is not aromatic because various types of ring strain destabilize an all-planar geometry. So, it is unable to adopt the necessary planar configuration.
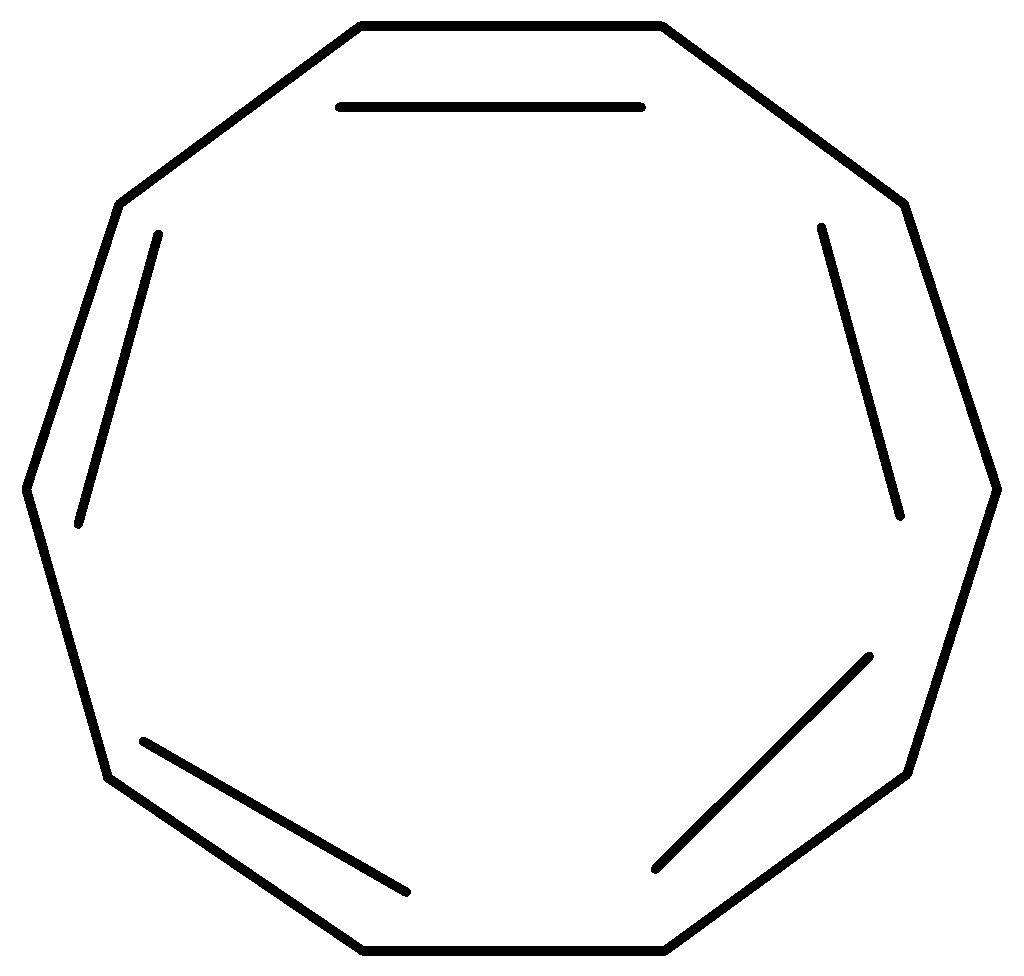
Figure: [10]-Annulene
In [12]-Annulene, the three H in-between the ring is far enough and do not create any strain for the planar arrangement. So, this is a cyclic, planar system having continuous delocalisation of π electrons and fulfilling the first condition. But the number of π electrons continuously delocalised are 12 i.e. 4nπ electrons, where n=3 . Since it is a 4nπ electron system, it is anti-aromatic in nature.
Figure: [12]-Annulene
Therefore, the correct answer is option (D).
Note: In [8]-Annulene, the regular planar octagon has bond angles of 135∘ with large bond angle strain due to large deviation from sp2 bond angles of 120∘ and to overcome the strain, molecule assumes a non-planar, tub-shaped geometry. Hence, it is non-aromatic.
