Question
Question: Which of the following amino acids is neutral? A.Aspartic acid B.Glycine C.Lysine D.Arginine...
Which of the following amino acids is neutral?
A.Aspartic acid
B.Glycine
C.Lysine
D.Arginine
Solution
Amino acids can be understood as the organic compounds which contain both the amine group as well as the carboxyl functional group along with a side chain that is specific to each amino acid. The key constituent elements of amino acids are nitrogen (N), oxygen (O), carbon (C) and hydrogen. Many times, other elements are also found in the side chains of amino acids.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Amino acids are usually classified on the basis of the characteristic properties of the side chains that are attached to them. If the side chain is a polar compound in nature, then that side chain makes the corresponding amino acid hydrophilic. On the other hand, if the side chain is a non – polar compound, then the side makes the corresponding amino acid hydrophobic. The side chains also cause the amino acids to be either weak acidic compounds or weak basic compounds.
This is dependent on whether the side chain itself is an acid or if it is a base. A basic side chain would induce basic character while an acidic side chain would induce an acidic character. The molecular structures of the given compounds are:
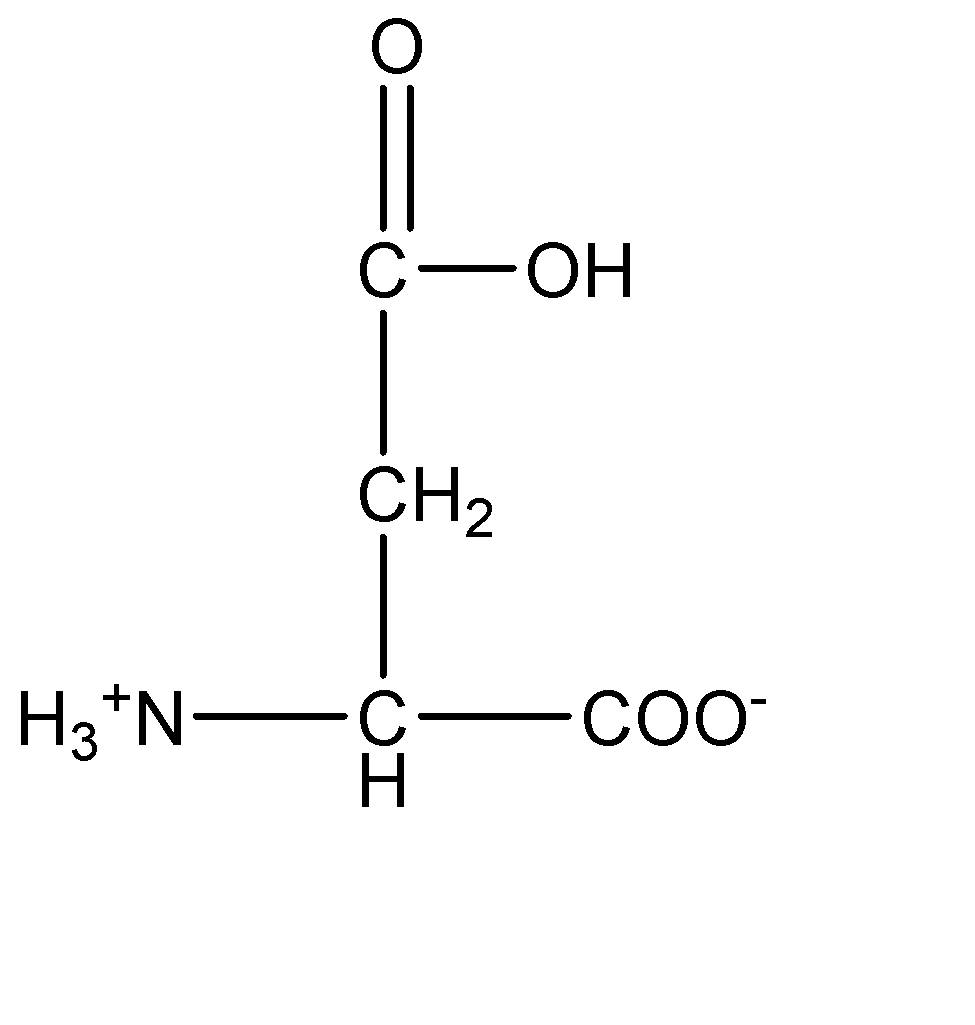
1.Aspartic acid:
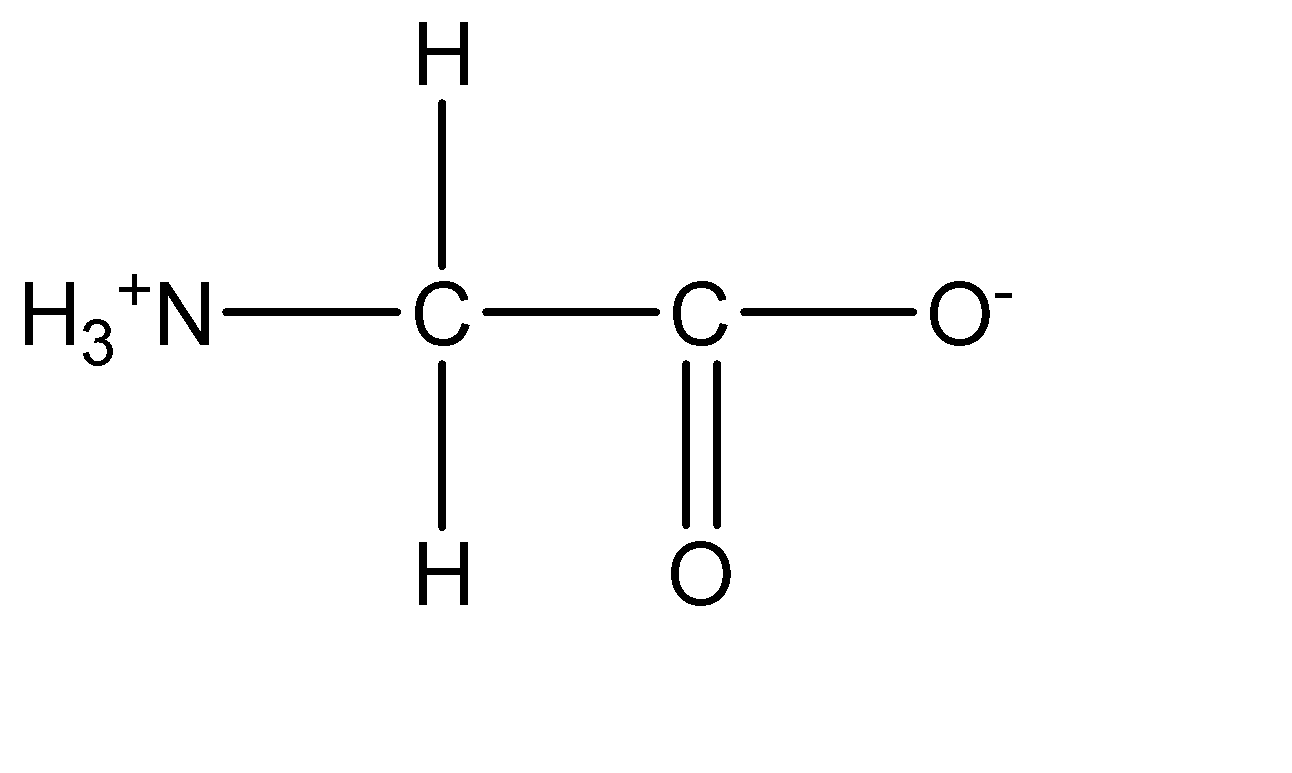
2.Glycine:
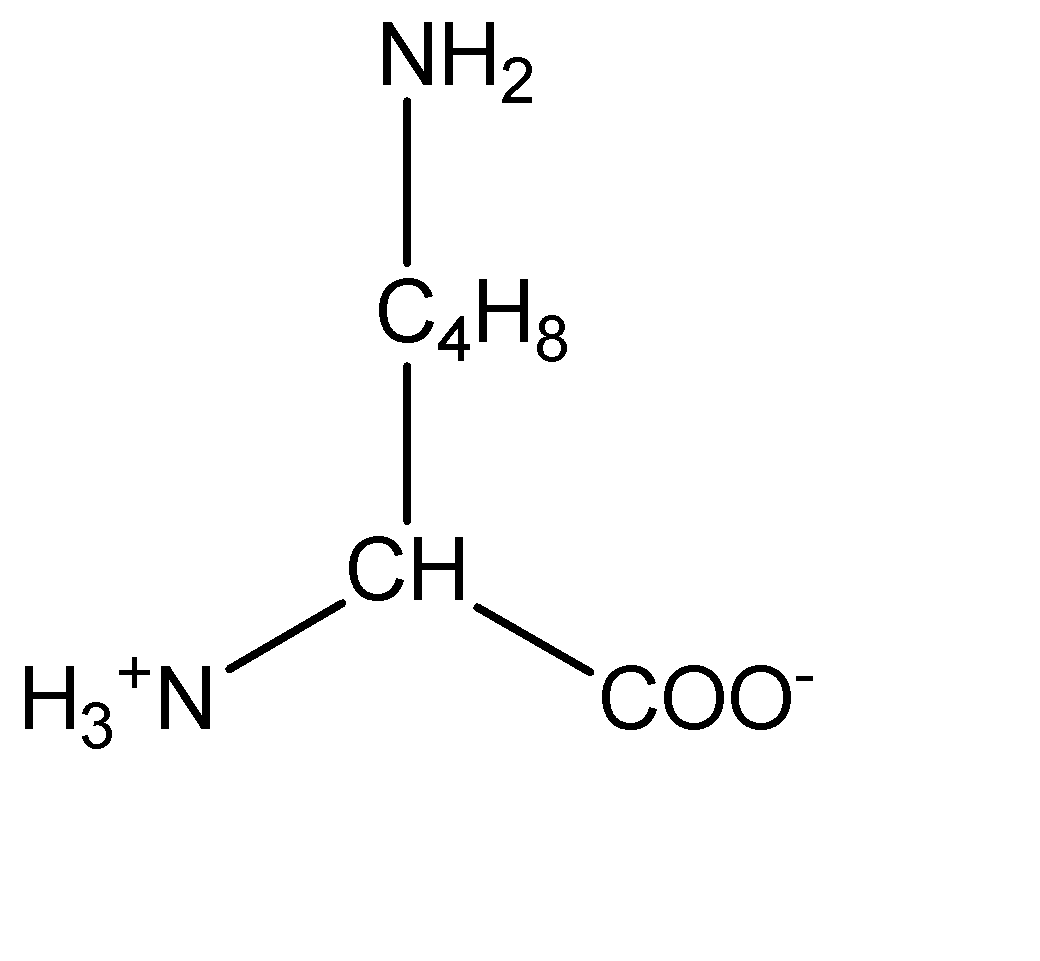
3.Lysine:
4.Arginine:
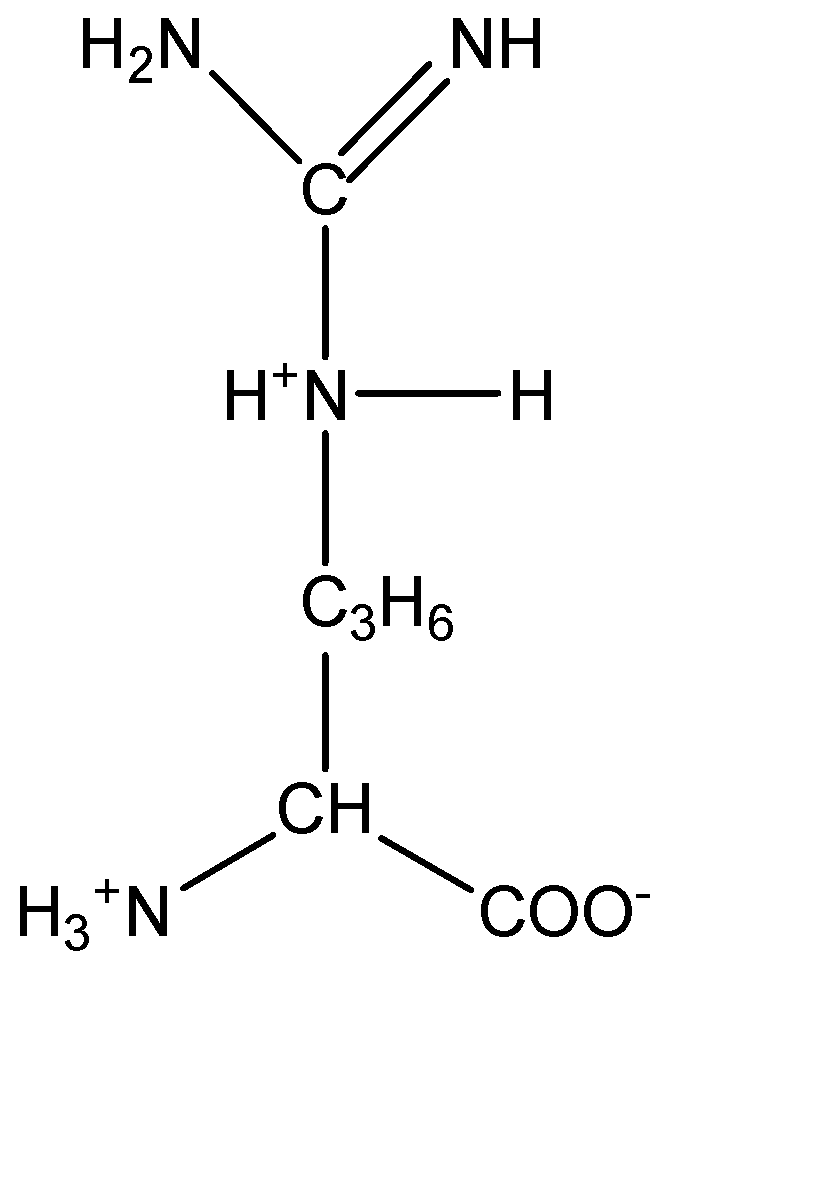
From the molecular structures above, we can observe that arginine has the basic amine group which induces a basic character on the compound. On the other hand, aspartic acid contains the acidic carboxylic group which induces acidic character on the amino acid.
Only glycine does not have any side chain that would influence its pH character. Hence, it is the only amino acid in the given options.
Hence, Option B is the correct option
Note: Glycine is a colourless, sweet-tasting crystalline solid. It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom.
