Question
Question: Which of the following alkanes cannot be synthesized by the wurt’s reaction in good yield? A. 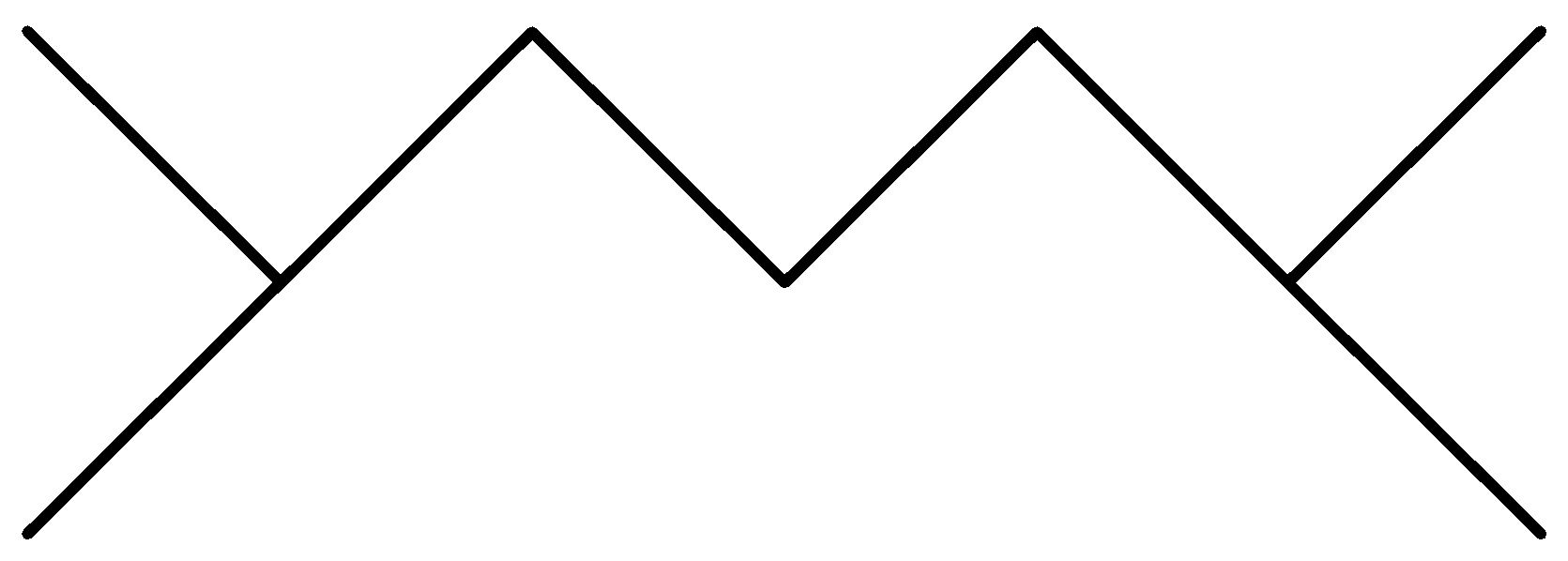
B.
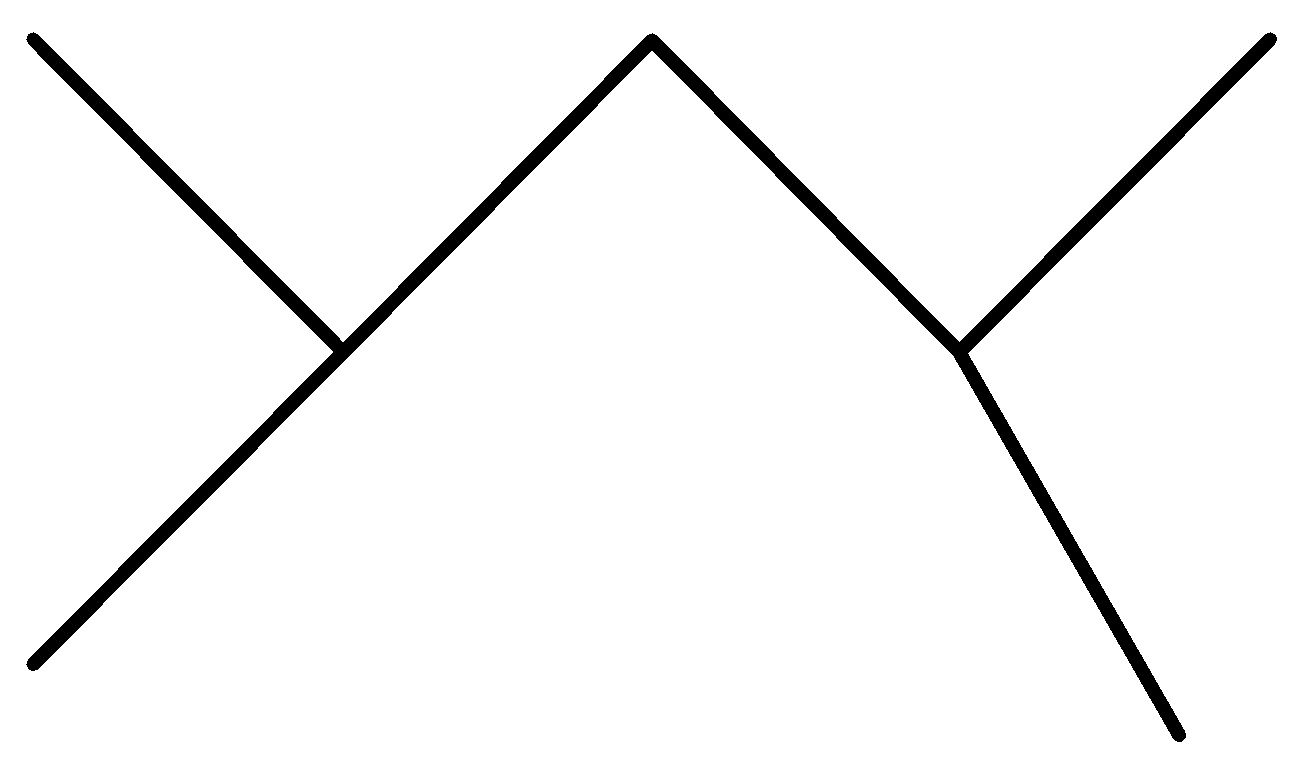
C.
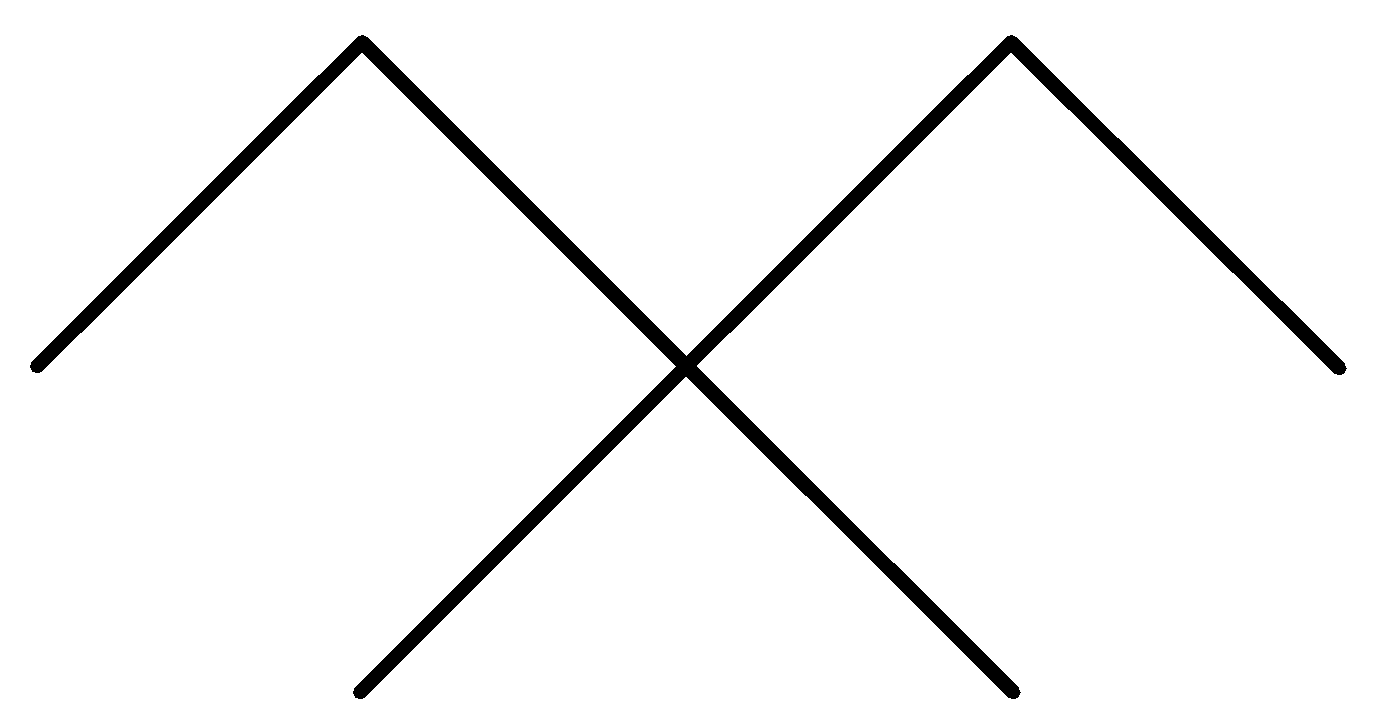
D.
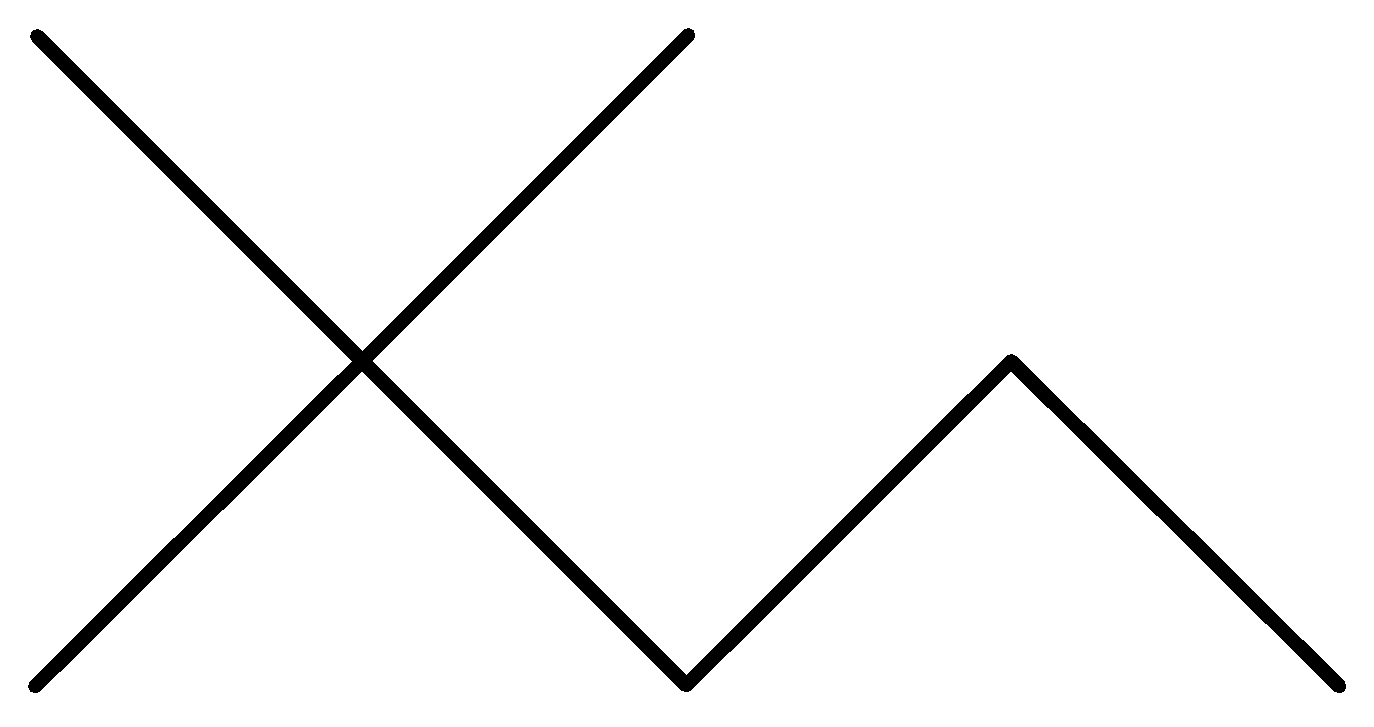
Solution
An organic chemical coupling reaction where the sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen is Wurtz reaction. The symmetrical alkane with an even number of the carbon atoms can be prepared by wurtz reaction.
Complete step by step answer:
In the wart’s reaction, two different alkyl halides are coupled to yield a longer alkane chain with the help of sodium metal and dry ether solution.
Other than sodium, metals like silver, indium, activated copper, zinc, and iron is also used in the Wurtz reaction in order to obtain alkanes.
This reaction involves free radicals which allows the possibility of side reactions that lead to the formation of alkenes as the product.
2R−X+2Na→R−R+2Na+X−
From the above equation, it can be observed that the two R groups are joined, which yield an alkane with a longer chain along with Next, where X is a Halogen.
In the above options, none of the alkanes are symmetrical,
Hence, None of them are synthesized by the wurt’s reaction in good yield
Note: Only symmetric alkanes can be synthesized through this method since a mixture of alkane products are formed when dissimilar alkanes are reacted. There exists a side reaction through which an alkene product is formed. It should be also noted that methane cannot be synthesized through the Wurtz reaction since the product of an organic coupling reaction must have at least two carbon atoms. A reaction that is related to the Wurtz Reaction in which aryl halides are used instead of alkyl halides is called the Wurtzilite reaction.
