Question
Question: Which of the following acid-base reactions is/are feasible? A. \({\text{PhONa}}\,\,{\text{ + }}\...
Which of the following acid-base reactions is/are feasible?
A. PhONa + CH3CH2OH→PhOH + CH3CH2ONa
B. PhOH + NaOH→PhONa + H2O
C.PhONa + aqHCl→PhOH + NaCl
Solution
The feasibility of acid-base reaction depends upon the strength of the acid. More strong acids favour the reaction in forward-direction whereas the weak acid does not.
Step by step answer: The strength of acid decides the feasibility of a reaction. A strong acid on dissociating forms conjugate base. If a strong acid is present on the reactant side it will dissociate easily and react with another reactant that is base.
The strength of acid is decided on the basis of the PKavalue. Higher the PKa value lower will be the strength of the acid.
So, the presence of strong acid on the reacting side favours the reaction in forward-direction.
So, the presence of strong acid on the product side favours the reaction in a backward direction.
The first reaction is represented as follows:
PhONa + CH3CH2OH→PhOH + CH3CH2ONa
Phenoxide reacting with ethanol forms phenol and ion of ethanol. Here, phenol is strong acid (PKa=10) than ethanol ((PKa=16)). Phenol is present on the product side, so the reaction will be favoured in the backward direction. So, the reaction (A) is not feasible. The option (A) is incorrect.
The second reaction is represented as follows:
PhOH + NaOH→PhONa + H2O
Phenol reacting with sodium hydroxide forms phenoxide and water. Here, phenol is strong acid(PKa=10) than water(PKa=15.7). Phenol is present on the reactant side, so the reaction will be favoured in the forward-direction. So, the reaction (B) is feasible. The option (B) is correct.
The third reaction is represented as follows:
PhONa + aqHCl→PhOH + NaCl
Phenoxide reacting with aqueous hydrogen chloride forms phenol and sodium chloride. Here, aqueous hydrogen chloride strong acid(PKa=−6.3) than phenol(PKa=10). Aqueous hydrogen chloride is present on the reactant side, so the reaction will be favoured in the forward-direction. So, the reaction (C) is feasible. The option (C) is correct.
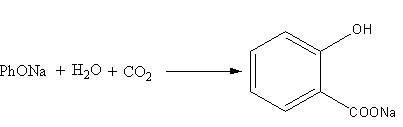
The fourth reaction is represented as follows:
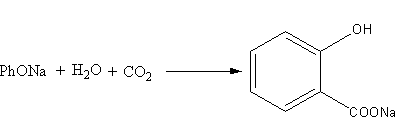
Phenoxide reacting with water and carbon dioxide forms ions of salicylic acid and sodium hydroxide. Here, phenol is strong acid (PKa=10) than salicylic acids because in salicylic acid hydrogen bonding is present. Phenol in the form of phenoxide ion is present on the reactant side, so the reaction will be favoured in the forward-direction. So, the reaction (D) is feasible. The option (D) is correct.
So, the reaction (B), (C) and (D) are feasible.
Therefore, option reaction (B), (C) and (D) are correct.
Note: The feasibility of the acid base reaction is compared on the basis of acid strength only, not the base. On comparing both confusions will create higher the PKa value high will be the strength of the base.
