Question
Question: Which of the compounds is chiral? A.
B.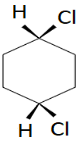
C.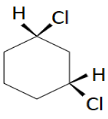
D.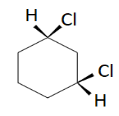
Solution
To identify the chiral compounds we should know some of the basic concepts such as chiral carbon, stereoisomers, enantiomers, symmetry and optical activity. A compound to be chiral should possess one or more chiral carbon atoms, there should be no symmetry, non-superimposable mirror images and should be optically active.
Complete answer:
A carbon atom whose valency is satisfied by 4 different groups or in other words a carbon atom attached to 4 different groups is known as a chiral carbon. The molecule which contains a chiral carbon is known to be a chiral molecule. The chiral molecule will have non-superimposable mirror images and should lack symmetry(asymmetric).
The isomers which have the same molecular and structural formula but differ in their spatial arrangement of atoms are known as stereoisomers. The stereoisomers show different physical and chemical properties.
The non-superimposable mirror images of a molecule are known as enantiomers.
A molecule which has a chiral carbon and is an enantiomer can rotate a plane polarized light. This is known as optical activity. If a molecule can rotate a plane polarized light either towards left or right is known to be optically active.
Let’s get into the options.
Trans-1,4-dichlorocyclohexane
This has a plane of symmetry and has no chiral carbon and is optically inactive. So, the molecule is achiral.
Cis-1,4-dichlorocyclohexane
Even this has no chiral carbon and is optically inactive. So, the molecule is achiral.
Trans-1,3-dichlorocyclohexane
This has two chiral carbon atoms and lacks a plane of symmetry. It has a non-superimposable mirror image and is optically active. Hence the molecule is chiral.
Cis-1,3-dichlorohexane
This has two chiral carbons but has a plane of symmetry that passes through Carbon 2 and 5. It is a meso compound hence optically inactive. So, the molecule is achiral.
Hence the correct answer is option(C).
Note:
The stereoisomers which differ only in the rotation of the plane polarised light are known as optical isomers. Optical isomers which rotate the plane polarised light towards the right side are called dextro rotatory(d) while those which rotate left are known as laevo rotatory(l).
