Question
Question: which of the amino groups in semicarbazide will react with carbonyl groups? 
(A) 1
(B) 2
(C) 3
(D) 1 & 3
Solution
The structure given in the equation is of semicarbazide and we can easily observe that there are two amino groups (−NH2) present in semicarbazide. As they are present in different electronic environments they will show different behavior while reacting with different groups. According to the question, we will predict which amino group in semicarbazide will react with the carbonyl group.
Complete step by step answer:
We will discuss all the three amine groups one by one which are numbered as (1),(2) and (3) . So let’s start one by one.
(1) In the first option we have given that (1) amino group (−NH2) will react with the carbonyl group (−C=O) . So, let’s study the environment of (1) amino group (−NH2) . So, we can observe that in semicarbazide (1) amino group (−NH2) is bonded with carbon only. In a highly acidic medium, the (−NH2) group gets protonated. Due to strong (−I) effect of the protonated group, the lone pair of electrons on the (−NH2) group of protonated hydrazine is not available for the nucleophilic attack on the (−C=O) group and hence hydrazine formation does not occur.
(2) The second (−NH) group will also not be available for the nucleophilic attack on the (−C=O) group and hence hydrazine formation does not occur.
(3) The third (−NH2) group which is the only reactive amine in semicarbazide. This (−NH2) group is attached to (−NH) group has a lone pair of electrons and also available for nucleophilic attack on carbonyl group. Therefore, it will lead to formation of semicarbazones.
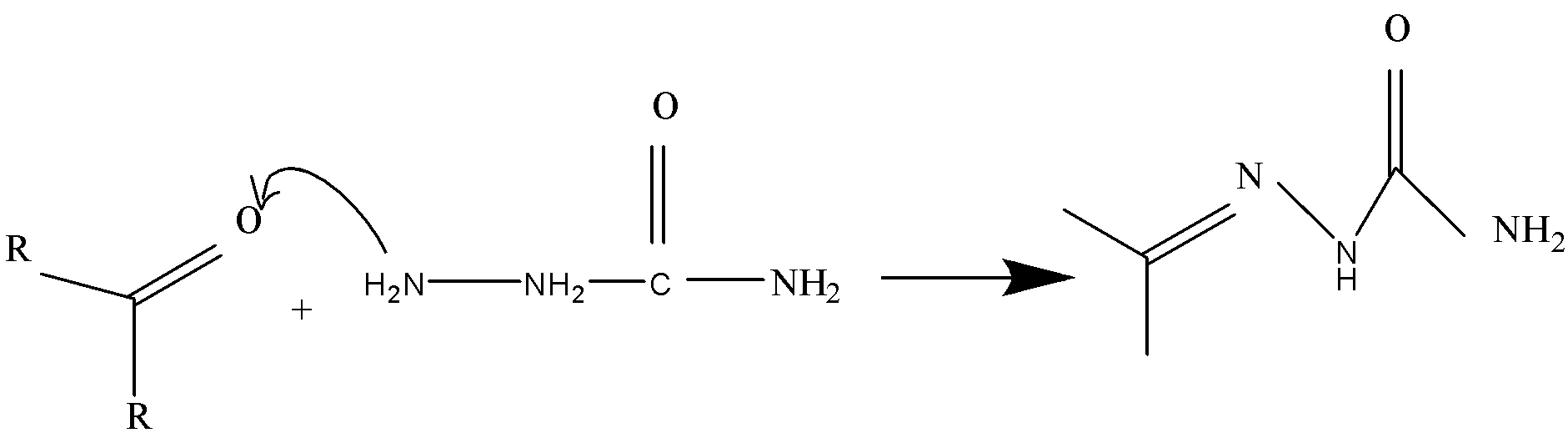
Final result: The correct option is, ‘(C) 3’.
Note: The (1) amino group (−NH2) is also called the amide functional group. It also has a lone pair on nitrogen atoms but the lone pair is involved in the resonance with the carbonyl group.
