Question
Question: Which of following reactions are shown with incorrect major product : $CH_3 - \begin{matrix} CH_3 \...
Which of following reactions are shown with incorrect major product :
CH3−CH3∣C∣CH3−ONa+CH3−Cl⟶CH3−CH3∣C∣CH3−O−CH3
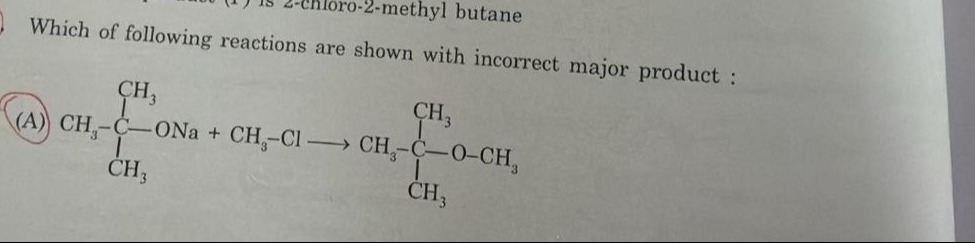
CH3−CH3∣C∣CH3−ONa+CH3−Cl⟶CH3−CH3∣C∣CH3−O−CH3
The product shown in the reaction is the correct major product.
Solution
The given reaction is a Williamson Ether Synthesis between sodium tert-butoxide and methyl chloride.
Sodium tert-butoxide is a strong, bulky base. Methyl chloride is a primary alkyl halide. The reaction proceeds via an SN2 mechanism.
The tert-butoxide ion acts as a nucleophile, attacking the carbon of methyl chloride and displacing the chloride ion.
CH3−CH3∣C∣CH3−O−+CH3−Cl⟶CH3−CH3∣C∣CH3−O−CH3+Cl−
Elimination (E2) is not favored because methyl chloride does not have beta-hydrogens. Therefore, the major product is tert-butyl methyl ether, which matches the product shown.
