Question
Question: Which Newman projection shows the most stable conformation of the following compound? 
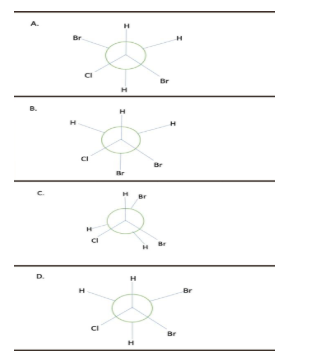
Solution
Anti staggered conformation is more stable as compared to gauche and eclipsed conformations because in it, the bulky groups are oppositely placed to each other, thus reducing the steric hindrance between the groups resulting in the stability of the molecule.
Complete step by step answer:
-In the Newman projection, the two carbon atoms of ethane, the one which is nearer is depicted as a dot and the rear carbon atom is represented as a circle. The three hydrogen atoms attached to each carbon atom are shown with the help of three lines either bulging out of the circle or diverging of the dotted lines. These lines are aligned at an angle of 120∘ to each other in case of every carbon.We can rotate the carbon-carbon bond as it results in many various possible molecular conformations. The lowest energy conformation of ethane is called the ‘staggered’ conformation. In this conformation, all of the C-H bonds on the front carbon are placed at an angle of 60 degrees with respect to the C-H bonds on the back carbon. This angle between a sigma bond on the front carbon and a sigma bond on the rear carbon is called the dihedral angle. In this conformation, the distance between the bonds is maximum and results in a more stable structure.
-Now on rotating the front methyl group to 60 degrees clockwise, the molecule is in the highest energy conformation and eclipsed as the hydrogens of the front carbon are as close as possible to the hydrogens on the back carbon. When one or more hydrogens are replaced by any other group, anti-staggered conformation becomes more stable as the bulky groups are inclined opposite to each other.
-Therefore, option A is most stable because the bulky groups (bromine) lie opposite to each other and form anti-staggered conformation. While in B and D, they are close to each other causing repulsion and less stability. Option C is eclipsed, so definitely high in energy and less stable than rest three conformers.
Hence, the correct option is (A).
Note:
An Ethane molecule has only two types of conformers in terms of stability. But in butane, two more conformers are possible- anti and gauche. When two methyl groups are furthest from each other, it is anti. When two methyl groups are at dihedral angles of 60 and 300 degrees, it is gauche.
