Question
Question: Which molecule has V-shape? This question has multiple correct options (A) \[{{\text{H}}_{\text{2...
Which molecule has V-shape? This question has multiple correct options
(A) H2O
(B) SnCl2
(C) PbC12
(D) None of these
Solution
In each molecule, determine the hybridization of the central atom. From the hybridization, determine the electron pair geometry and the molecular geometry.
Complete step-by-step answer:
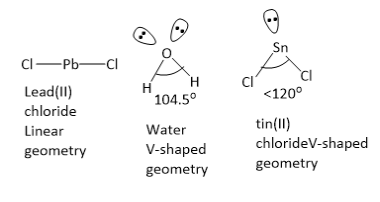
PbC12 molecule has linear geometry. In water molecules, the central oxygen atom has 2 bond pairs and 2 lone pairs of electrons. Oxygen atom is sp3 hybridized with tetrahedral electron pair geometry and V shaped molecular geometry. The ideal tetrahedral bond angle is 109o28’ , but in water molecule, the H−O−H bond angle is 104.5o . This is due to greater lone pair lone pair repulsion and lone pair bond pair repulsion as compared to bond pair bond pair repulsion.
In SnCl2 molecule, the central tin atom has 2 bond pairs and 1 lone pair of electrons. Tin atom is sp2 hybridized with Trigonal planar electron pair geometry and V shaped molecular geometry. The ideal bond angle is 120o , but in SnCl2 molecule, the Cl−Sn−Cl bond angle is less 120o . This is due to greater lone pair bond pair repulsion as compared to bond pair bond pair repulsion. The molecules H2Oand SnCl2 have V-shaped geometries.
Hence, the options (A) and (B) are the correct answers.
Note: For several molecules, the electron pair geometry is different from the molecular geometry. This is due to the presence of lone pairs of electrons on the central atom.
