Question
Question: Which L-sugar on oxidation gives an optically active dibasic acid (\[2 - COOH\] groups)? A.?
A.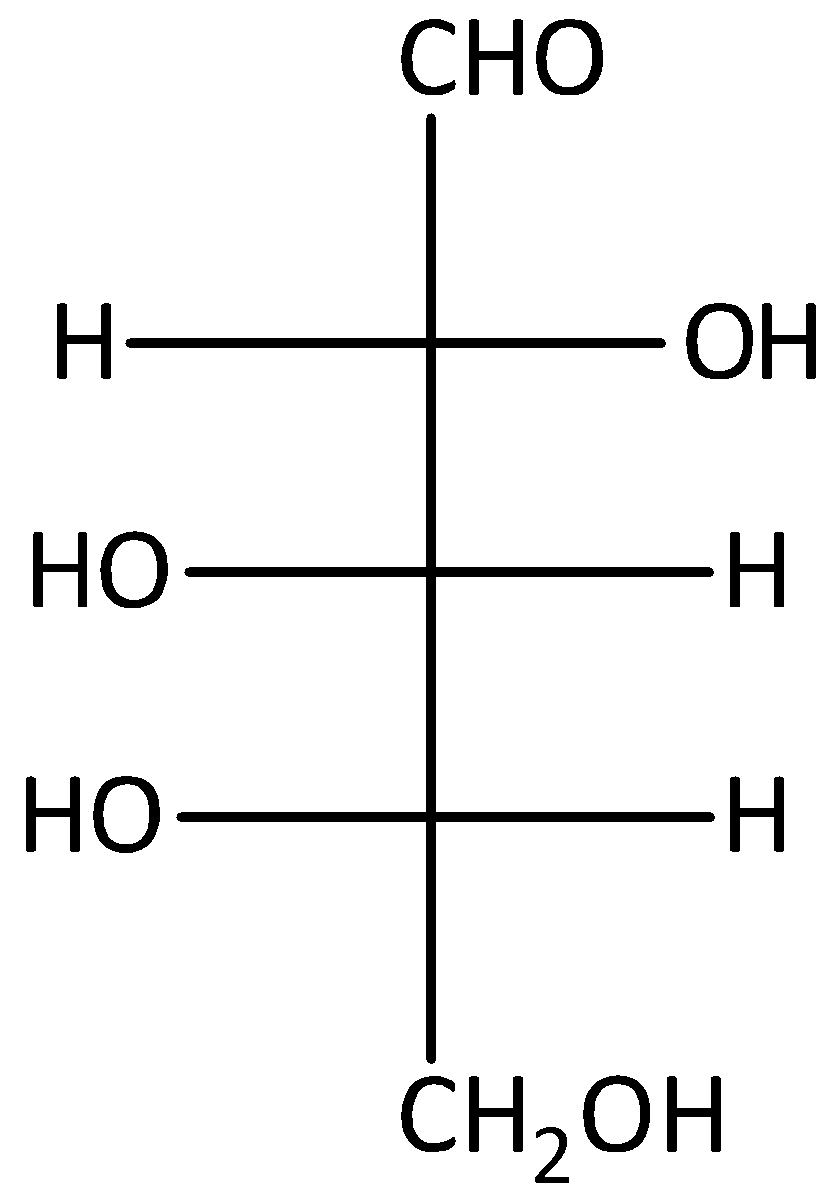
B.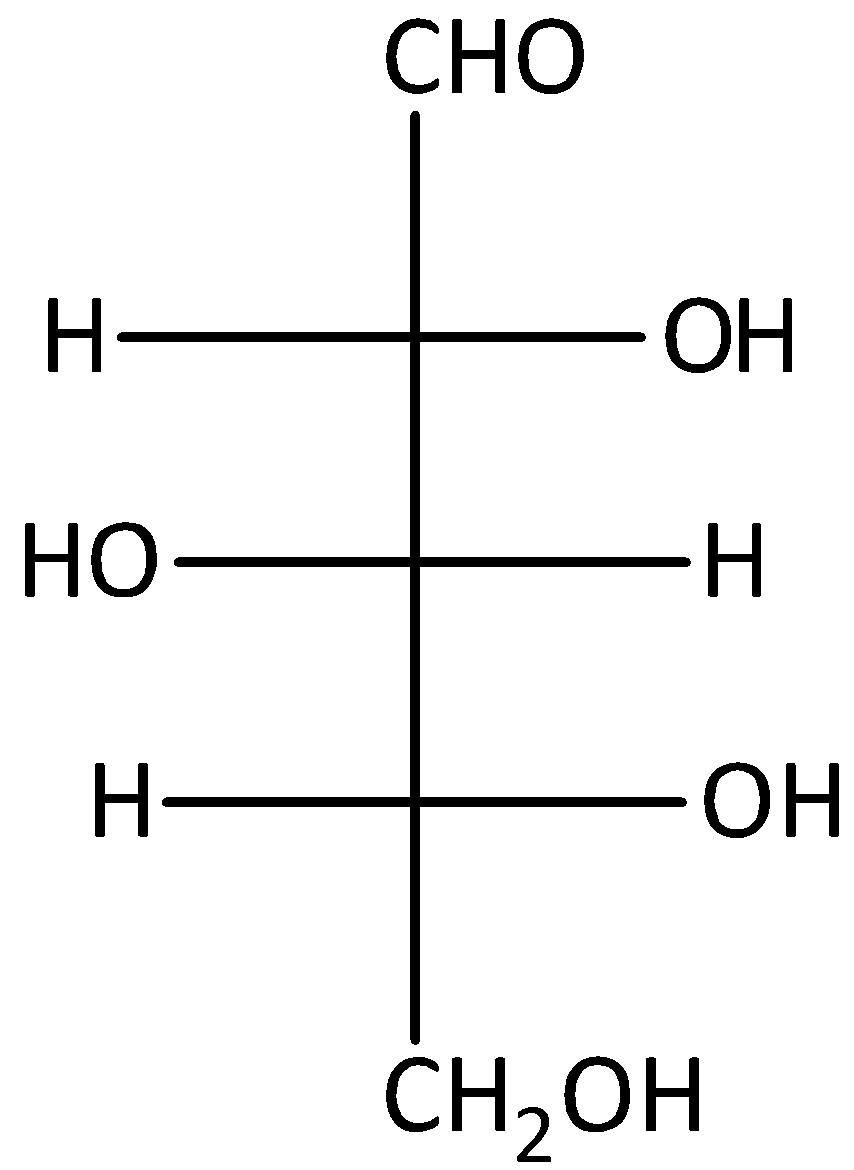
C.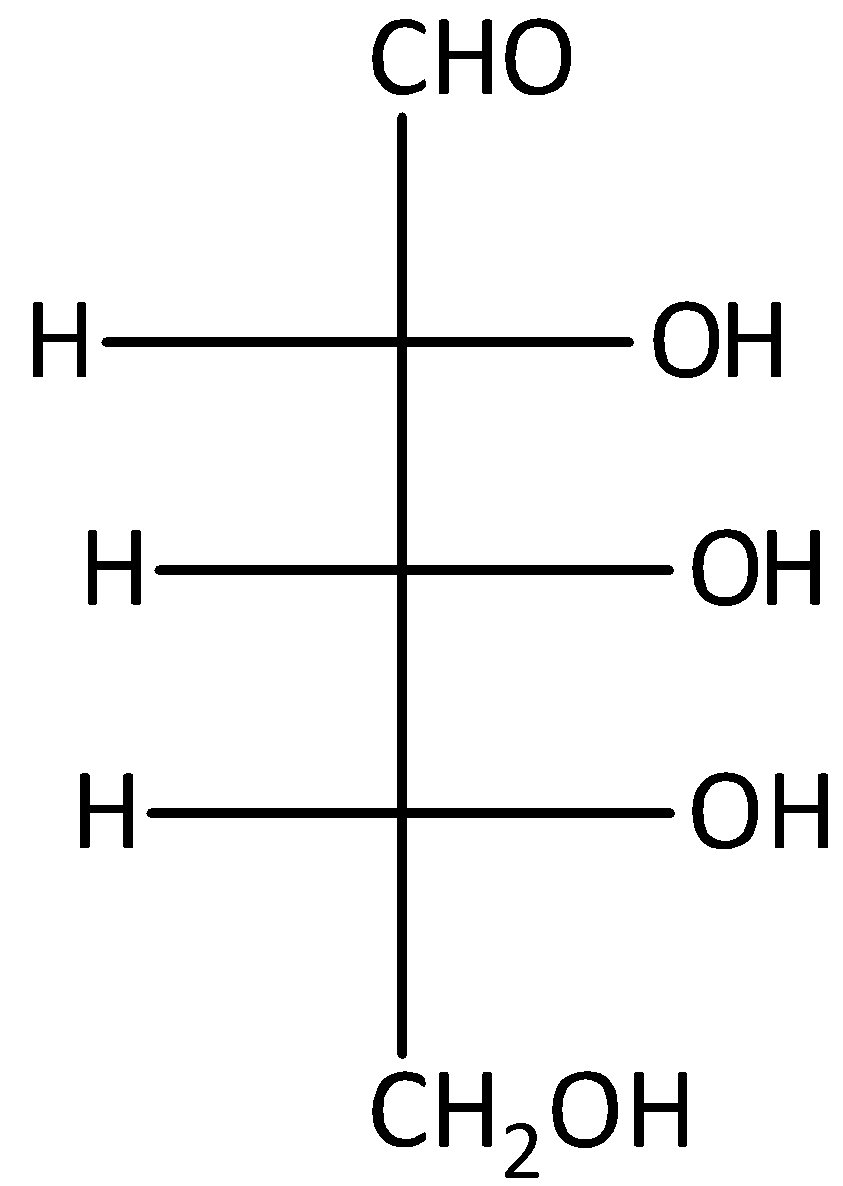
D.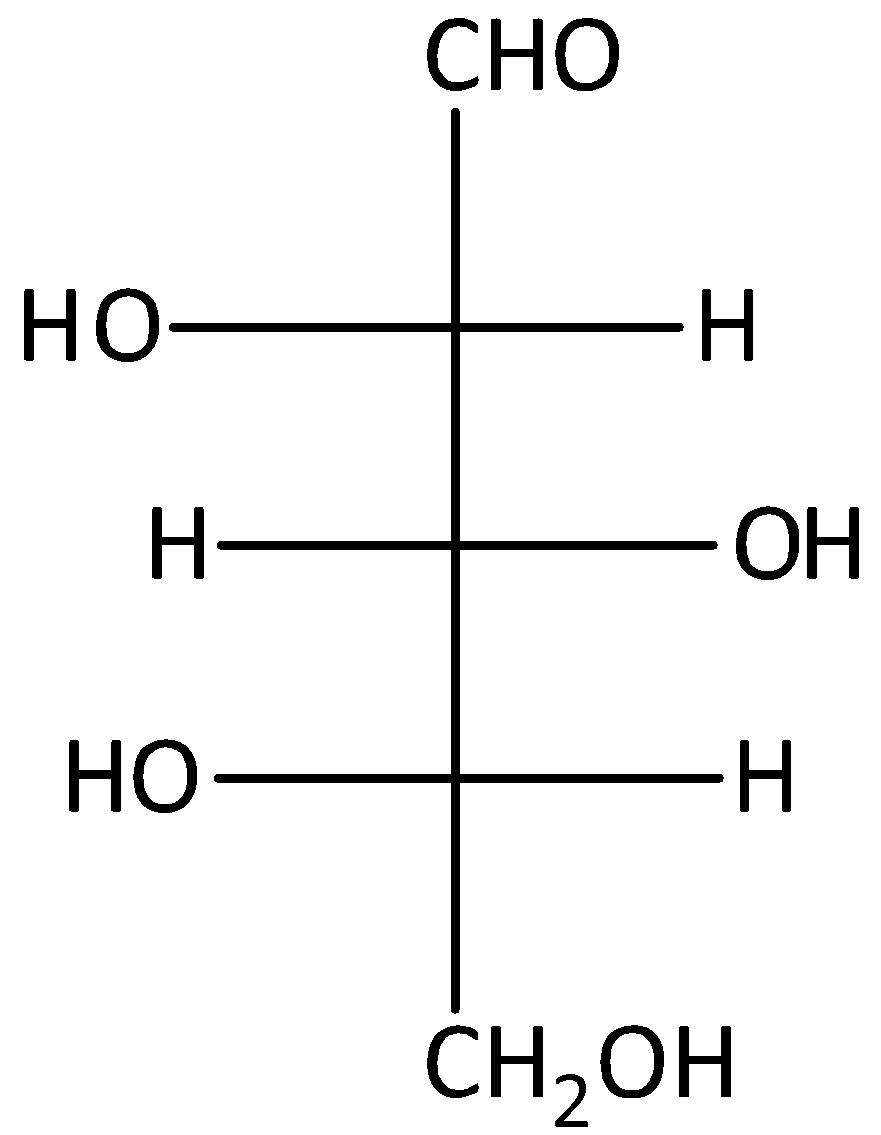
Solution
We need to know that the L-glucose is an organic compound having the formula, C6H12O6 or it also written as, O=CH[CH]5H. The L-glucose is the l-isomer of the glucose. Naturally it will not occur in higher living organisms. And the molar mass of the l-glucose is equal to 180.6g/mol. And it can be prepared by using D-glucose, by a convenient method and it can easily be prepared in the laboratory. The D-glucose is found in vegetables and plants naturally. The l-glucose is the primary source of energy in the living organism.
Complete answer:
It will give an optically active dibasic acid by undergoing oxidation. If the sugar is optically active, when it does not contain any plane of symmetry. According to the given options, option (A) and (D) are given the L-sugars after oxidation. But compounds (B) and (C) are D-sugars after oxidation. And the compound (A) gives the optically active mirror image on oxidation. Let’s see the reaction,
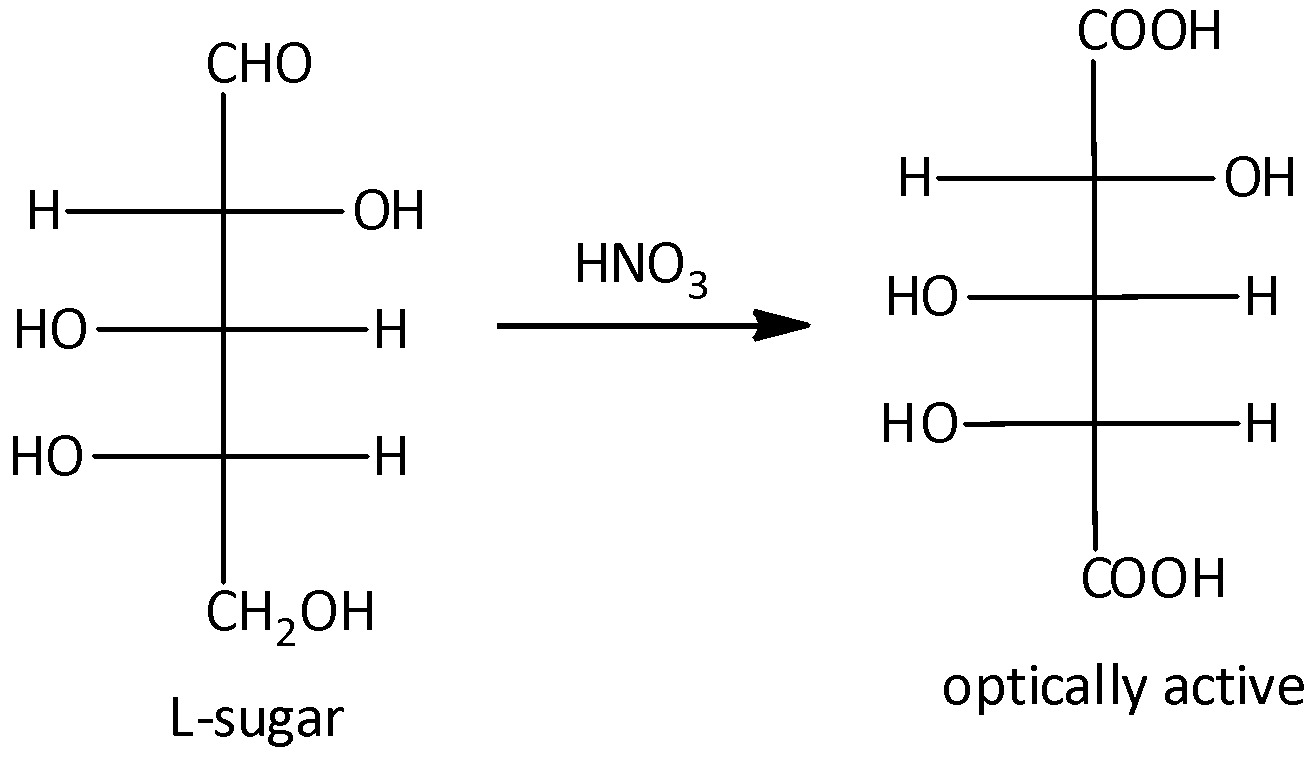
Hence, option (A) is correct.
The given compound B is D-glucose. Therefore, it will not give optically active dibasic acid on oxidation. Hence, option (B) is incorrect.
The compound C is D-glucose. Therefore, it will not give optically active dibasic acid on oxidation. Hence, option (C) is incorrect.
The given compound D is L-glucose. But, it will not give optically active dibasic acid on oxidation. Hence, option (D) is incorrect.
Hence, option (A) is correct.
Note:
We have to remember that the oxidation – reduction reaction is also known as redox reaction. This is a type of chemical reaction that undergoes the transfer of electrons between two species. During a chemical reaction, the oxidation state of an atom, molecule or ion may change. It may increase or decrease the oxidation number by losing or gaining the electrons.
