Question
Question: Which kind of point defect is found in \[KCl\] crystal ? A. Frenkel B. Schottky C. Linear D....
Which kind of point defect is found in KCl crystal ?
A. Frenkel
B. Schottky
C. Linear
D. Impurity
Solution
There are three types of point defects ie stoichiometric defect, Frenkle defect and schottky defect. These are the imperfections in crystal lattice due to missing atoms or ions, it creates a vacancy for extra atoms or ions.
Complete step by step answer: KCl is an ionic compound, having small difference in size of cations and anions present in the crystal lattice.
In such compounds, more examples :
NaCl,KBr,CsClandAgBr,the point defect seen is schottky defect. Schottky defects consist of cations and anions in stoichiometric ratio that are unoccupied.
Simple structure of ionic crystal is A−B+.
This defect was named after walter H schottky, who demonstrated that in crystal lattice, oppositely charged ions ie. Cation and anion leave their sites and get incorporated at surface for instance and create oppositely charged vacancies in stoichiometric units and maintain overall neutral charge on ionic solid .
Two dimensional diagram of this defeat for KCl is :-
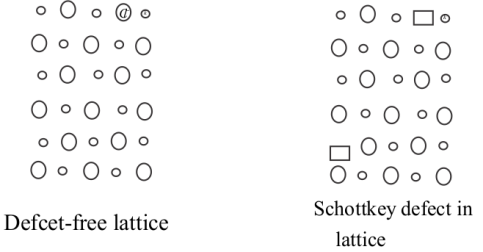
Due to schottky defect, the density of the solid crystal lattice becomes lower than actual theoretical density.
So, the correct answer is “Option B”.
Note: Both anion and cation leave the solid crystal and permanently move out of it. Generally , the number of Vacancies formed is two.
The number of schottky defects can be calculated by using the formula
ns=Next(2RT−ΔHs)
Where, ns= number of Schottky defects per unit volume.
R=gas constant
ΔH=enthalpy of defect formation
T= Temperature in kelvin.
