Question
Question: Which isomers of hexane give maximum number of stereoisomers on monochlorination A. n-hexane B. ...
Which isomers of hexane give maximum number of stereoisomers on monochlorination
A. n-hexane
B. 2-methylpentane
C. 3-methylpentane
D. 2,2-dimethylbutane
Solution
Number of stereoisomers depends on the number of types of carbon atoms and its substituents, such as methyl, methylene and so on. More the different type of carbon atom, more will be the number of stereoisomers
Complete step by step answer:
Stereoisomers are isomers with respect to the three dimensional orientation of the molecules.
Stereoisomers can be the basis of the position of the substituent groups to each other.
Number of stereoisomers increases, when the number of carbon atoms which can have stereoisomers increases. This means if a molecule has different types of carbon atoms, more likely it is that the molecule will have stereoisomers.
Let us draw the structures
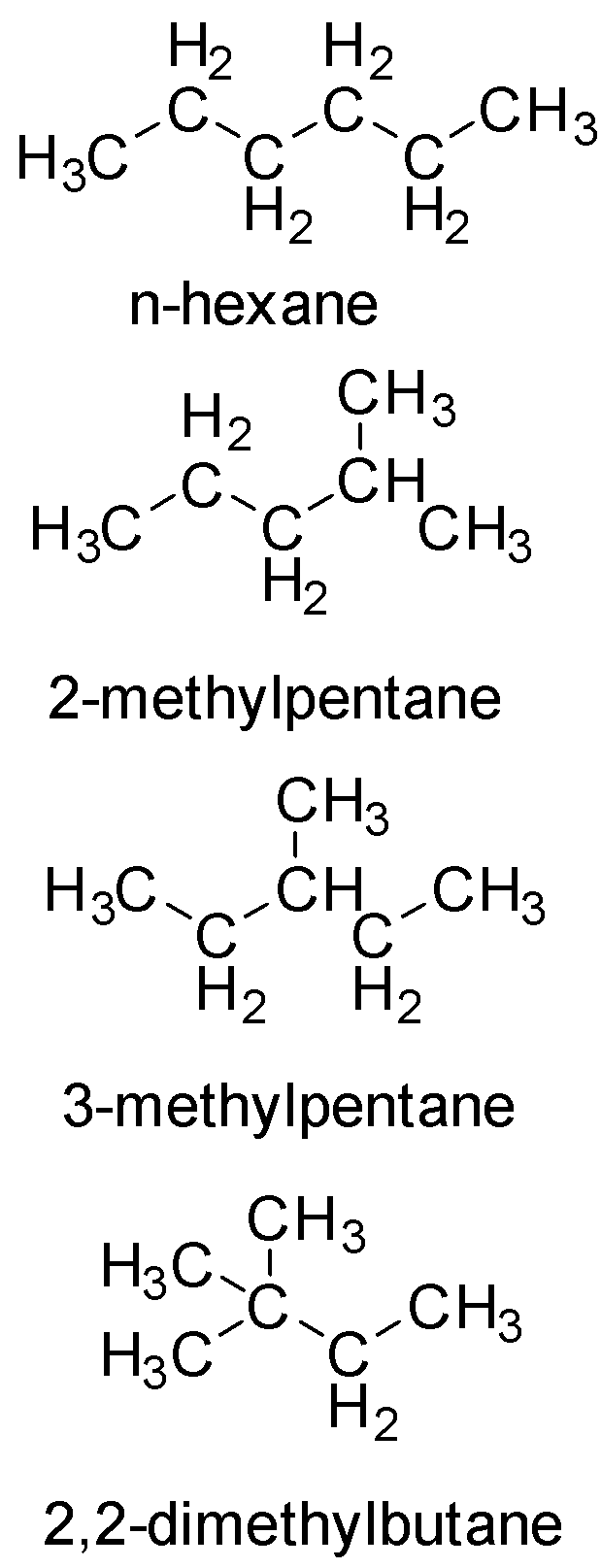
In the given option, 2-methylpentane has the maximum number of different types of carbon atoms
Hence, It will have the maximum possible value for stereoisomers
So, the correct answer is Option B.
Note: Monochlorination is a free radical process. It involves a homolytic cleavage of the bond, as opposed to the more common heterolytic cleavage of the bond. This gives rise to a free radical. Free radicals are much more potent than ions and have no charge so are unaffected by neighbouring species which are charged.
Free radicals are also very unstable and only exist for a few femtoseconds hence they need to be produced in situ, which means during the course of the reaction.
Peroxide initiators are good sources of initiating a free radical reaction, however the most preferred source, especially during halogenation is sunlight.
