Question
Question: Which is/are correct statements : (1) When 100 ml of 0.1 M NaCN solution is titrated with 0.1 M HCl...
Which is/are correct statements :
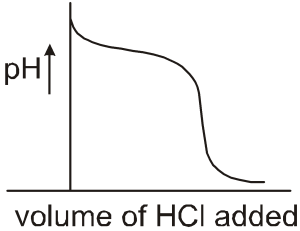
(1) When 100 ml of 0.1 M NaCN solution is titrated with 0.1 M HCl solution the variation of pH of solution with volume of HCl added will be (as shown in figure):
(2) Variation of degree of dissociation a with concentration for a weak electrolyte at a particular temperature is best represented by (as shown in figure):

(3) 0.1 M acetic acid solution is titrated against 0.1 M NaOH solution. The difference in pH between 1/4 and 3/4 stages of neutralization of acid will be 2 log 3.
a& c
b & c
a, b & c
b only
a, b & c
Solution
(1) Initially pH will decrease fast, then slowly due to buffer formation and then will decrease fast as buffer action diminishes.
(2) For a weak electrolyte
Ka = (1−α)Cα2
when a << 1 then a = CKaas C increases
⇒ a decreases
as C is tending to zero ⇒ a will be unity
(3) At 1/4th neutralization
CH3COOH + NaOH ⟶ CH3COONa + H2O
(0.1×43) (0.1×41)
pH = pKa + log [CH3COOH][CH3COO−]= pKa + log (31)
At 3/4th neutralization
pH = pKa + log 3
so difference in pH = D(pH) = log 3 – log = 2 log 3
