Question
Question: Which is the wrong statement about oxymercuration-demercuration? (a)- In the first step, oxymercur...
Which is the wrong statement about oxymercuration-demercuration?
(a)- In the first step, oxymercuration i.e., water and Hg(OAc)2 add to the double bond.
(b)- In the second step, demercuration i.e., NaBH4 reduces (−HgOAc)2 group to hydrogen
(c)- The net reaction is the addition of water according to Markovnikov’s rule.
(d)- Rearrangement takes place.
Solution
In all the steps there is a nucleophilic attack taking place. Oxymercuration-demercuration takes place in the presence of mercuric acetate. There is no formation of carbocation intermediates.
Complete answer:
Oxymercuration-demercuration is a reaction which is used for the synthesis of alcohols.
This reaction takes place according to Markovnikov’s rule which states that reagents would add to the unsymmetrical alkenes in such a way that the negative part of the adding molecule goes to the carbon atom of the double bond which has lesser number of hydrogen atoms.
Oxymercuration-demercuration: Alkenes react with mercuric acetate, (CH3COO)2Hg or Hg(OAc)2 , to form oxymercuration products which upon reduction with NaBH4 in basic medium gives alcohols. Thus,
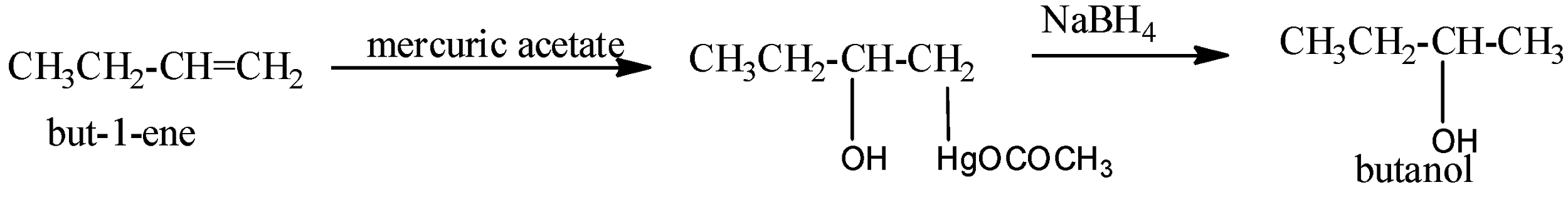
This two-step procedure is called oxymercuration-demercuration or oxymercuration-reduction and gives alcohol corresponding to Markovnikov’s addition of water to alkenes without any rearrangement since carbocation is not the intermediates.
Mechanism: Attack of π−electrons of the double bond of the alkene on mercuric acetate gives an unsymmetrical π−complex (I) with the expulsion of an acetate ion. Nucleophilic attack by H2O on the carbon atom of the π−complex carrying the +ve charge gives II which subsequently loses a proton to give the oxymercuration product (III). Another nucleophilic attack on III by hydride ion (:H−) from NaBH4 on the carbon atom carrying HgOCOCH3 group ultimately completes the addition with the expulsion of mercurous acetate.
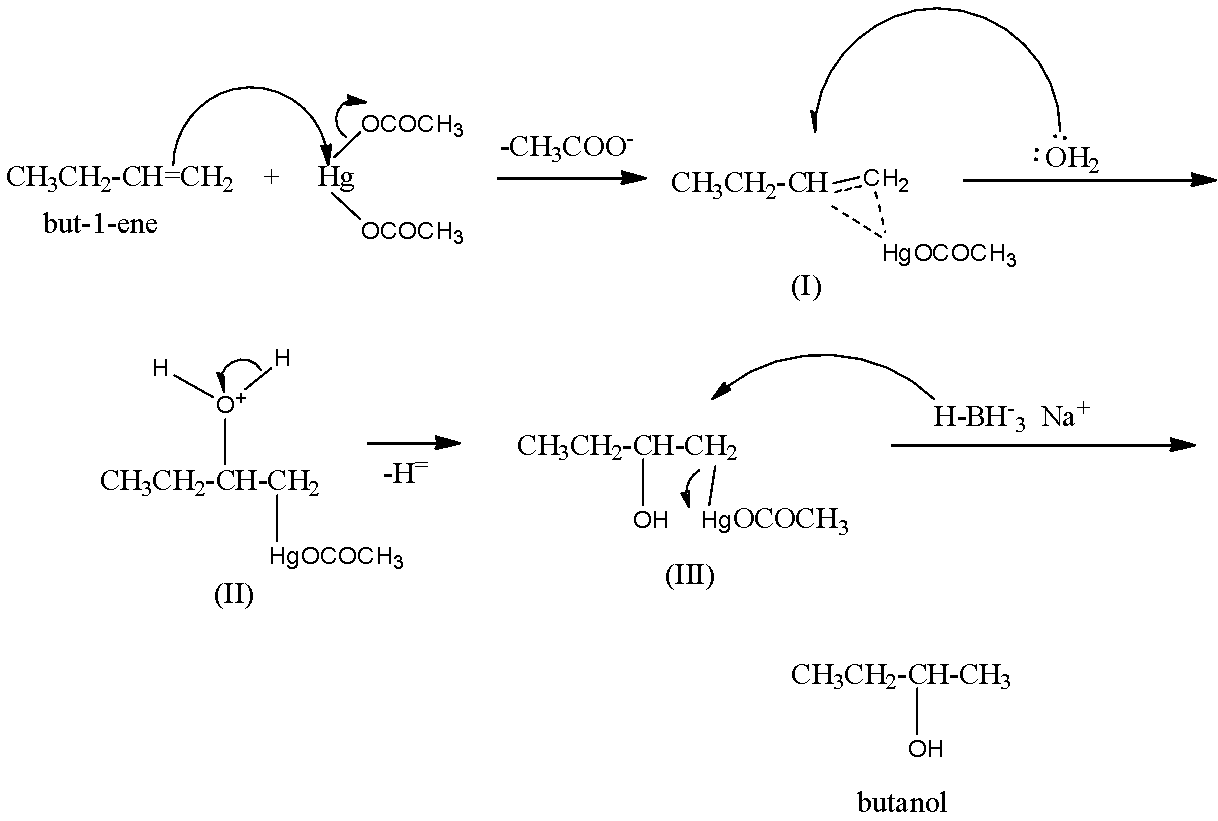
Hence, option (d) is incorrect.
Note: Hydration of alkene occurs through a carbocation intermediate. But hydroboration- oxidation and oxymercuration-reduction do not involve carbocation intermediates and hence always give unexpected or unarranged alcohols.
