Question
Question: Which is the unit of polarizability of a molecule? A) \({{\text{C}}^2}{{\text{m}}^{\text{1}}}{{\te...
Which is the unit of polarizability of a molecule?
A) C2m1N - 1
B) C2m - 1N1
C) C−2m1N - 1
D) C2m - 1N - 1
Solution
Polarizability determines the response of a molecule to an external field.
Formula Used: Polarizability is given by, α=Ep , where p represents the electric dipole moment of the molecule and E is the incident electric field.
Complete step by step answer:
Step 1: Define polarizability of a molecule.
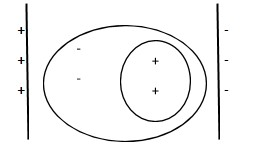
When a molecule is placed in an electric field of strength E a charge separation of electrons and positive charges takes place. This causes the formation of a dipole moment p inside the molecule. It is called induced dipole moment.
The induced dipole moment p is directly proportional to strength of the electric field E
i.e., p∝E or, p=αE .
where, α is the constant of proportionality called polarizability.
Thus, polarizability of the molecule is defined as the dipole moment induced in the molecule by an electric field of unit strength. It determines the relative tendency or dynamic response of the molecule when it gets distorted by an external electrical field.
Step 2: Express the relation for polarizability in terms of the units of the induced dipole moment and electric field strength.
We have, p=αE .
This implies that α=Ep .
The unit of dipole moment is C1m1and that of electric field strength is C−1N1 .
Substituting these units in the relation for polarizability we get, α=C−1N1C1m1 .
Finally, we have the unit of polarizability as C2m1N - 1 .
Therefore, the correct option is a) C2m1N - 1 .
Note: Charge separation of electrons and positive charges lead to the creation of electric dipoles (an electric dipole is a couple of opposite charges separated by some distance) and thereby induce a dipole moment inside the molecule. Electric field is the force by charge. Thus, has a unit of N1C−1 .
