Question
Question: Which is the smallest wavelength that will be observed in the spectra of \({ H }_{ e }^{ + }\) ion?...
Which is the smallest wavelength that will be observed in the spectra of He+ ion?
Solution
The smallest wavelength in the spectra of atoms corresponds to the Lyman series of the spectral series. The atomic number of He+ion is 2.
Formula used:
λ1=RZ2(n121−n221)
where
R is the Rydberg constant
Z is the atomic number
n1 is the orbit to which the electron jumps. n1=1,2,3,...∞
n2 is the orbit from which the electron jumps. n2=n1+1,n1+2,n1+3,...∞
λ is the wavelength of the corresponding spectral line
Complete step-by-step answer:
When electric current is passed through an atomic gas, the gas emits radiations of certain specific wavelengths. A spectrum is formed and this is called the Line emission spectrum. In this spectrum, groups of spectral lines are observed. These groups are named as spectral series. According to increasing wavelength, these series are named as Lyman series, Blamer series, Paschen series, Brackett series and Pfund series.
When electric current is passed through He+ ion, we observe all the spectral series mentioned above. Each spectral line in a spectral series corresponds to a certain wavelength. To obtain the smallest wavelength in the series, we have to concentrate on the Lyman series because this series comes first in the increasing order of wavelengths in the spectrum. We also have to go for the excitation from infinity to the first orbit to obtain the smallest wavelength out of the different excitations happening in the Lyman series. The following diagram explains the same.
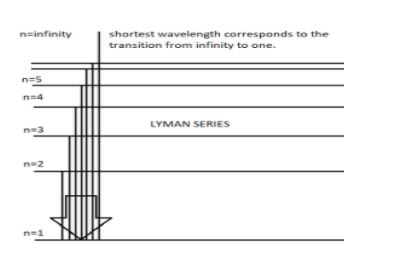
Rydberg equation is given by:
λ1=RZ2(n121−n221)
Here,
R=1.0987×107m−1
Z=2 (atomic number of He+ion is 2)
n1=1 (for Lyman series)
n2=∞ (for smallest wavelength)
Substituting the values given above, we have:
