Question
Question: Which is the most aromatic in the following? A.
B.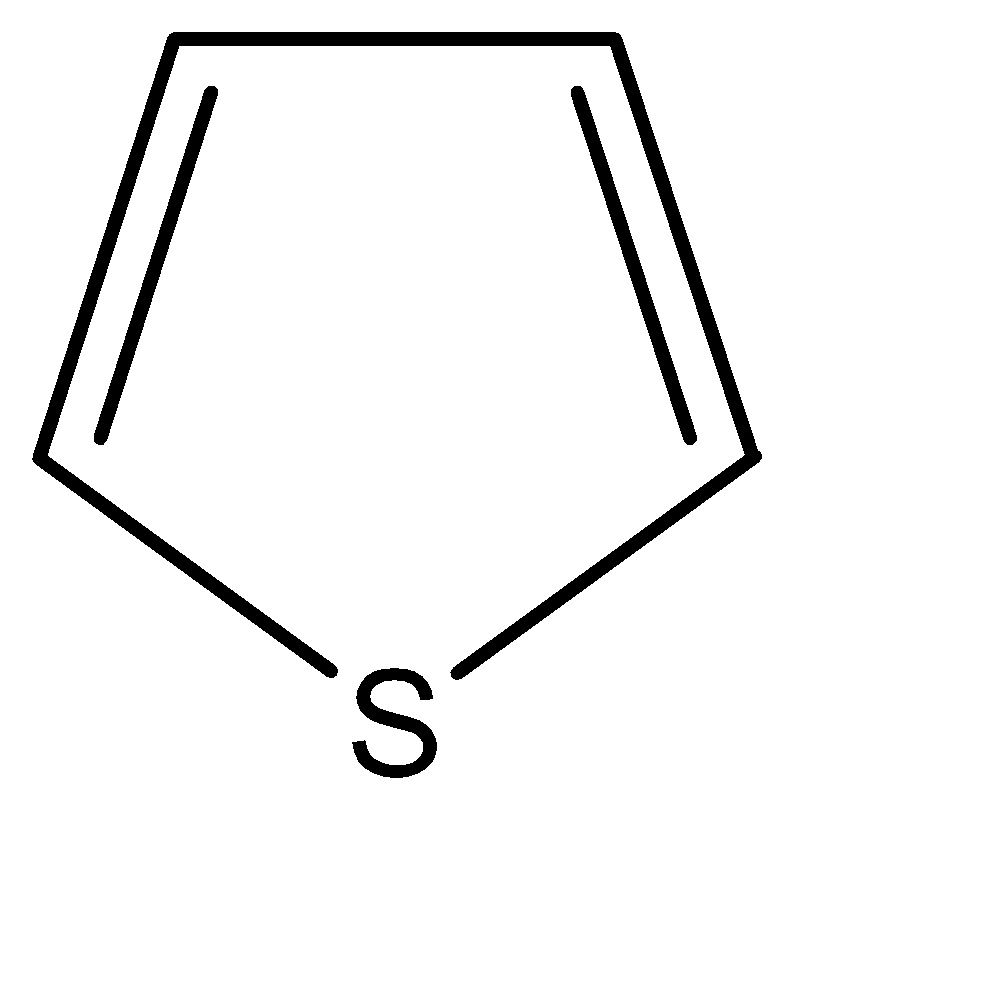
C.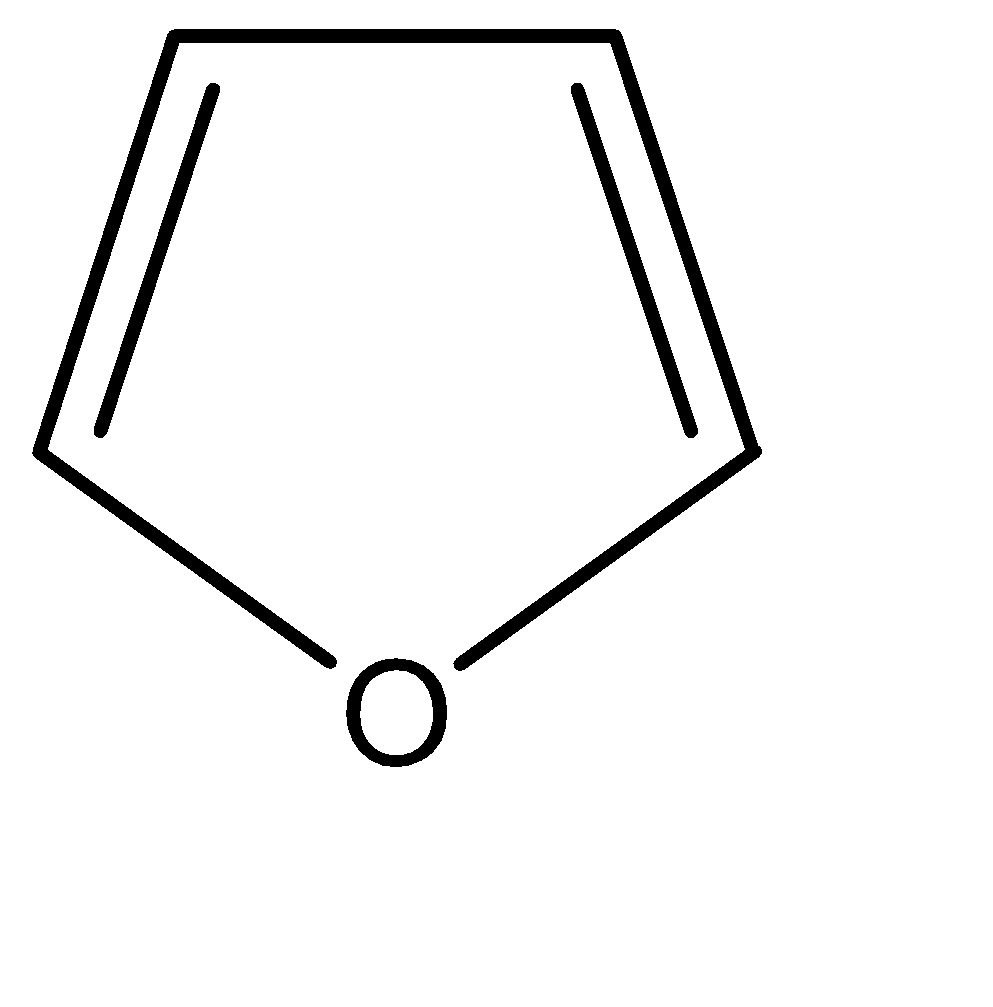
D.All having some aromatic character
Solution
To solve this question, we must understand whether the given compounds are aromatic in the first place. After that we must understand the trends that help in determining the degree of aromaticity and then compare the given compounds accordingly.
Complete step by step answer:
-All the given compounds are heterocyclic compounds, which means that in place of carbon atoms in a traditional organic cyclic compound, we place some other element. The most commonly used elements in place of carbon are nitrogen, oxygen, sulphur, among many others.
In order for a compound to be considered aromatic in nature, it must obey Huckel's Rule.
Huckle’s rule states that the number of electrons present in a compound should be equal to (4n+2) π , where n represents any natural number, and is not limited to any specific set of values. Now, π electrons can be understood as the electrons that are present in the π bonds of a double bond or a triple bond or in a conjugated p – orbital.
As we can observe in the given options, the number of π bonds in any compound is only 2. Hence the maximum number of π electrons present are 4. But this problem is countered by the unshared lone pairs of electrons on the hetero atoms in the compounds.
If we observe the electronic configurations and the number of bonds formed, we can say that number of free electrons on these hetero – atoms is as follows: N = 2, O = 4, S = 4. Now, that all these compounds have 6 π electrons (including π bonds and lone pairs), the only deciding factor for degree of aromaticity is the electronegativity of the hetero atoms. The heteroatoms being more electronegative than carbon, they pull the electron cloud towards themselves. Thus, there is an uneven charge distribution. Hence higher the electronegativity, lesser is the aromaticity of the compound. The order of negativity for the given compounds can be identified as: O>N>S
Hence, the compound containing sulphur is the most aromatic
Hence, Option B is the correct option.
Note:
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. Chemical compounds containing such rings are also referred to as furans. Furan is a colourless, flammable, highly volatile liquid with a boiling point close to room temperature.
