Question
Question: Which is the metamer of compound P? 
Solution
Aromatic hydrocarbons show structural isomerism due to the attachment to various functional groups. Metamerism is one of the types of chain isomerism or position isomerism which is observed with the same functional group and different alkyl groups attached to hetero atom or a benzene ring.
Complete step by step solution:
The phenomenon of existence with the same molecule formula of two or more compounds but different properties is known as isomerism and which compounds exhibit this phenomenon is called isomers.
The following flow chart shows different types of isomerism,
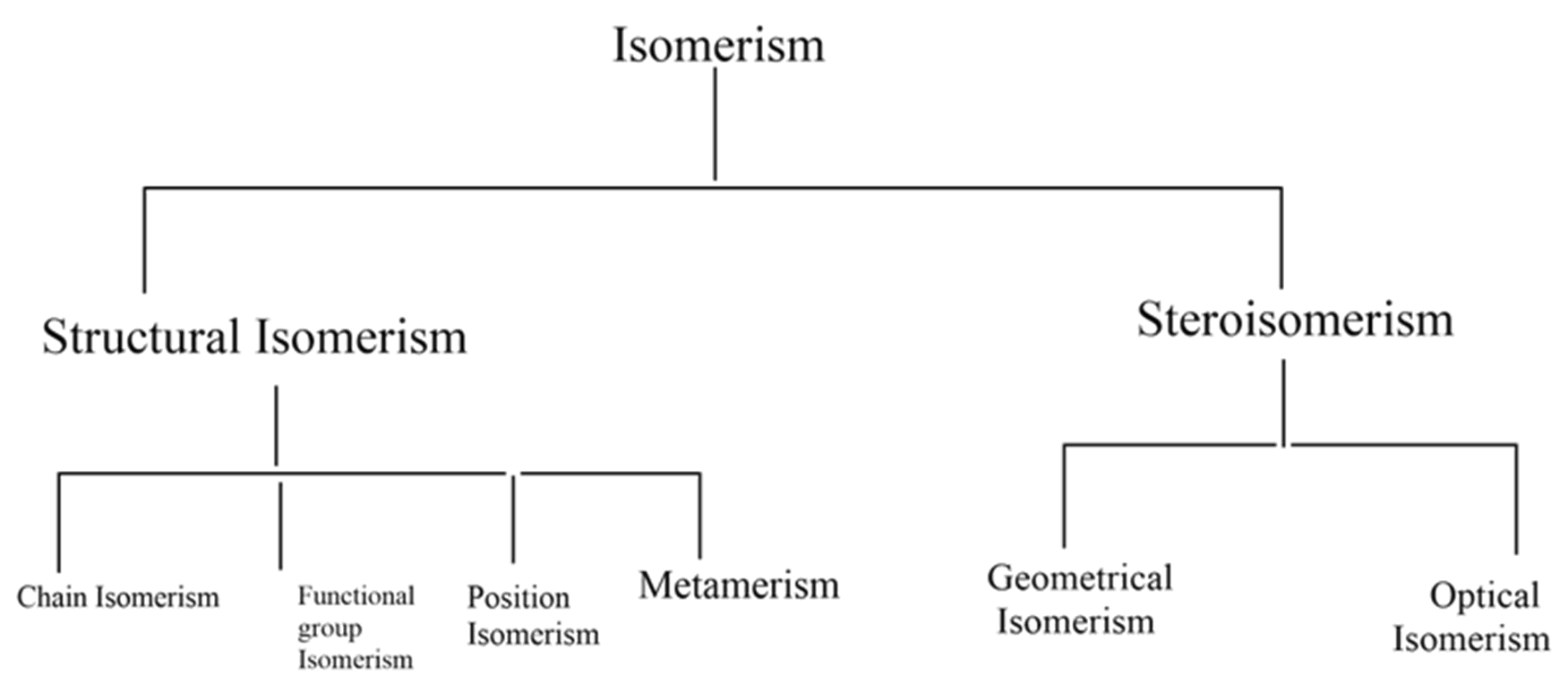
Structural isomerism: compounds with different structures with the same molecular formula are classified as structural isomers. Different types of structural isomerism shown in the flow chart are,
(1) Chain isomerism
(2) Position isomerism
(3) Functional group isomerism
(4) Metamerism
Chain isomerism: when compounds exhibit the same formula with different carbon skeleton are formed this isomerism. Mostly alkenes exhibit this type of isomerism
Position isomerism: when a substituted atom differs in the position in the carbon skeleton exhibits this type of isomerism. When alkenes or alkynes are involved besides reaction, the products show this isomerism.
Functional group isomerism: The compound with different functional groups having the same molecular formula exhibits this type of isomerism. For example, aldehyde, and ketones exhibit this isomerism with the same molecular formula.
Metamerism: this isomerism observed in compounds having the same molecular formula but different alkyl chains on either side of the functional group of the molecule.
The metamer of the given compound ‘P’ is,
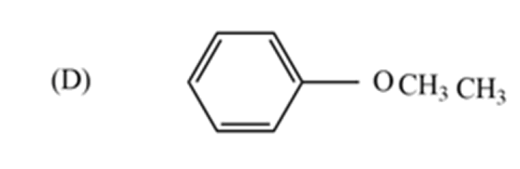
Because all other options are functional group isomers. In option (D), the given compound −CH3 And−OCH3 functional groups exhibit metamerism.
Note: Metamerism occurs among the members of the same homologous family. Sometimes metamers are also called position isomers. The compound which exhibits the metamerism having the same molecular weight but the difference in their chemical properties.
