Question
Question: Which is the final main product of the following reaction of trans-\[1,2\] -dibromocyclohexane? 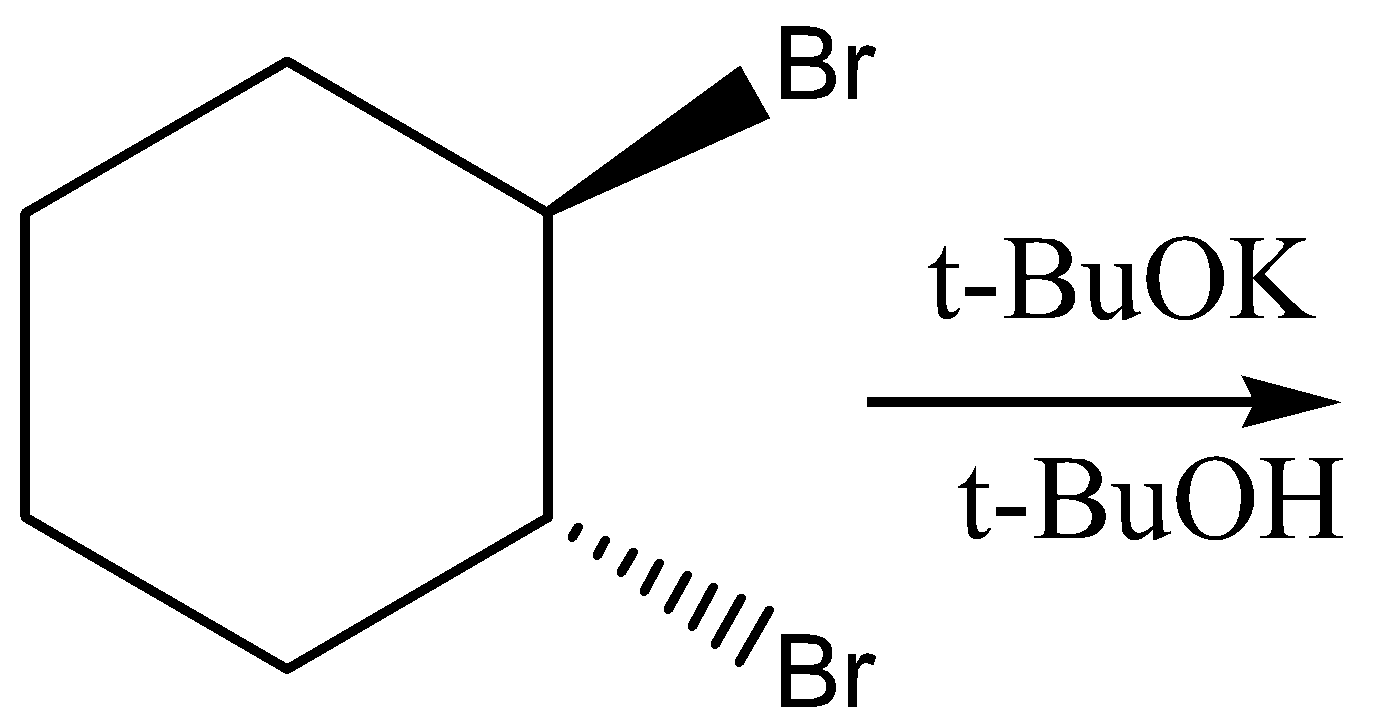
A.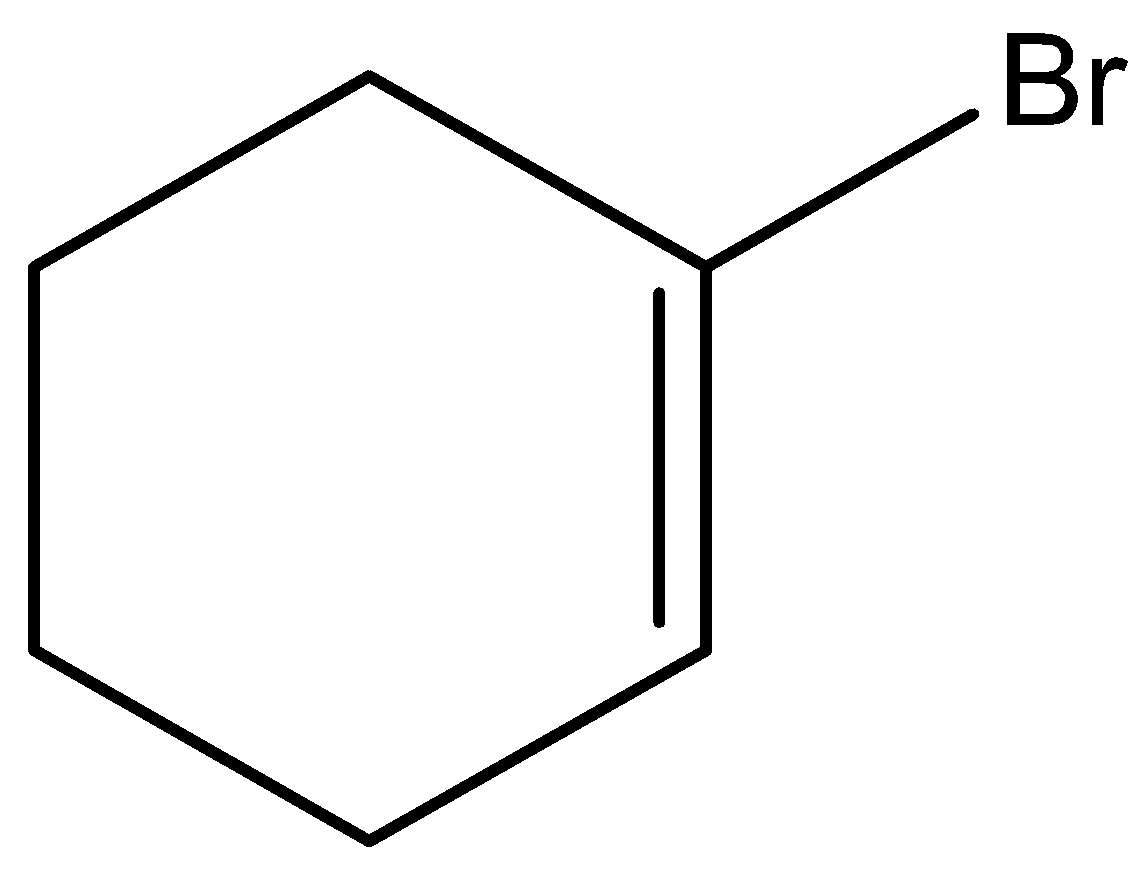
B.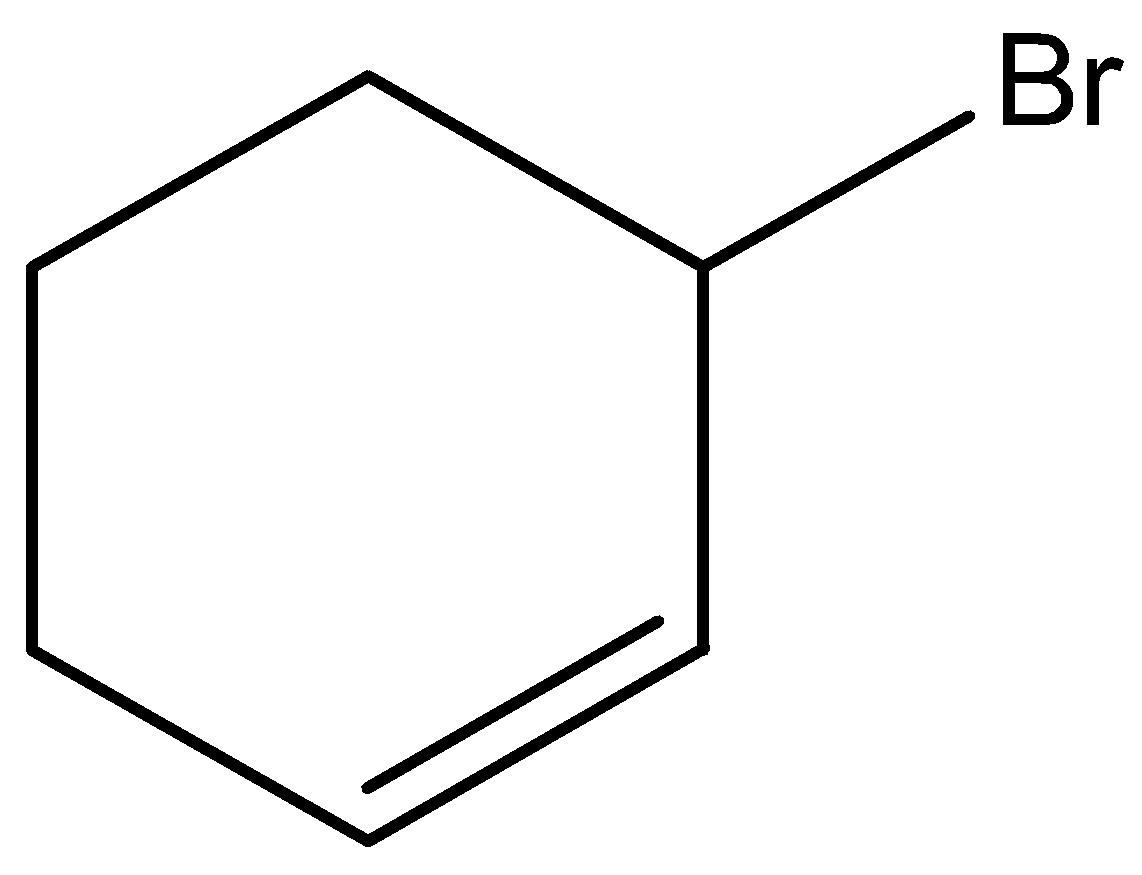
C.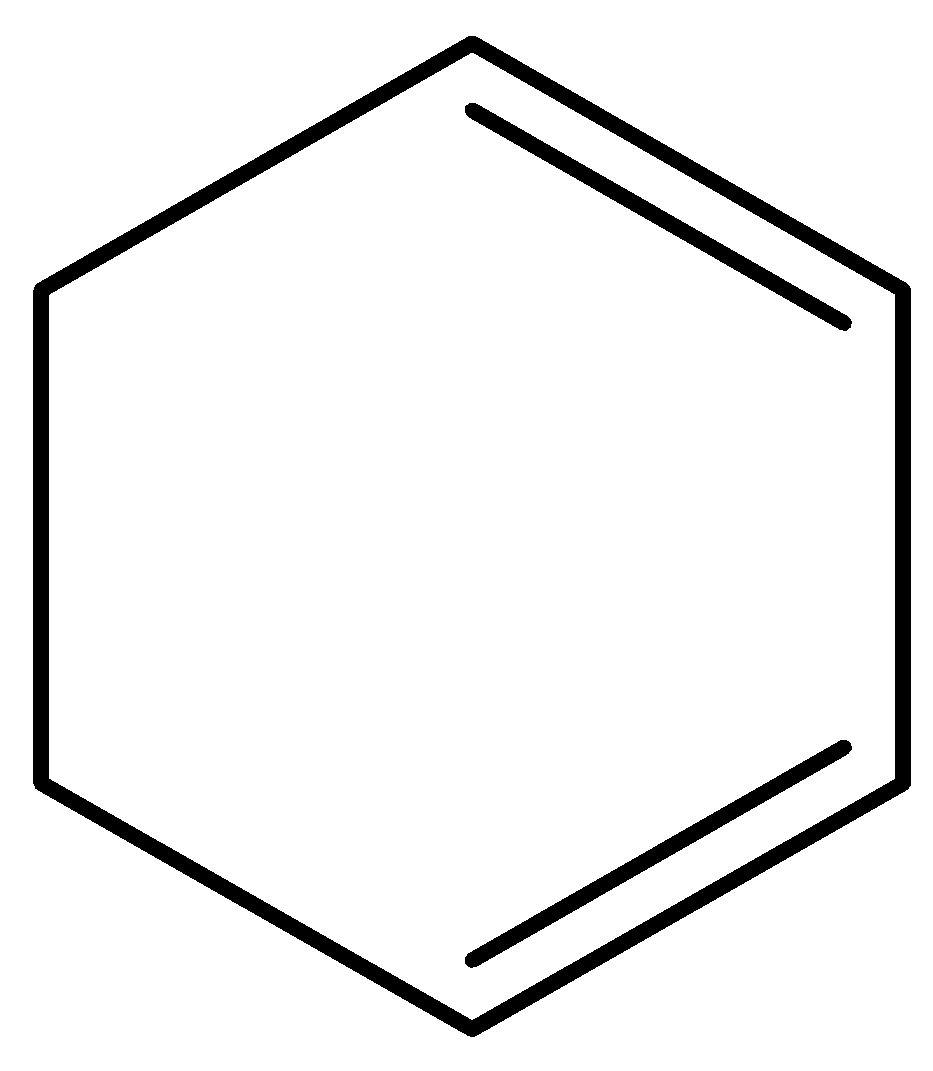
D.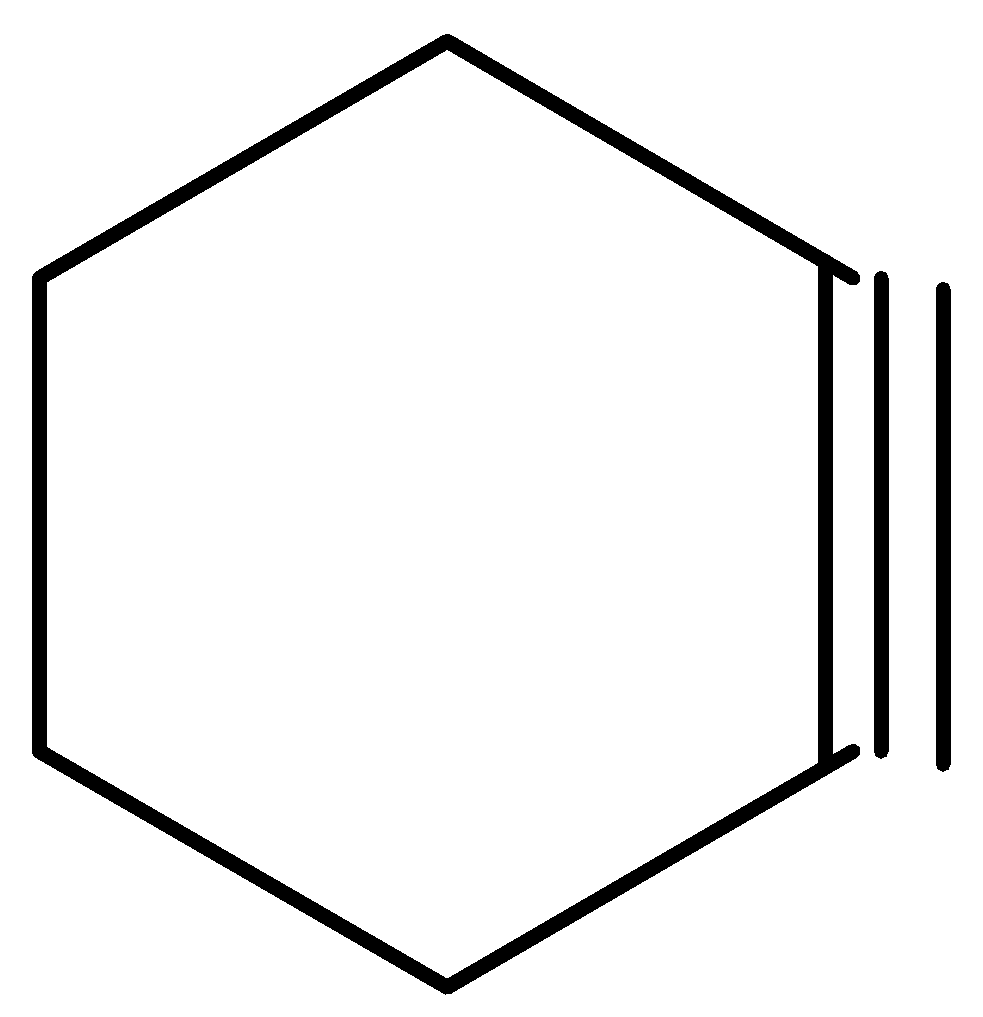
Solution
We have to remember that the cyclohexane is a polycyclic hydrocarbon consisting of a ring of six carbon atoms, a cyclic type of hexane used in the manufacture of nylon as a raw material. As a non-polar solvent, it does have a function. It is a cyclo-alkane and an organic volatile compound.
The "cis" and "trans" prefixes are from Latin: respectively, "this side of" and "the other side of". Cis suggests that the functional groups are on the same side of the carbon chain in the sense of chemistry, while trans indicates that functional groups are on opposite sides of the carbon chain.
Complete step by step answer:
We need to remember that a nucleofuge is a leaving group that preserves the lone pair with another species from its previous bond. For instance, a nucleophile attacks an organic compound that contains the nucleofuge in the SN2 system, which simultaneously breaks the bond with the nucleofuge.
When the H−C bond and C−LG bonds involved are coplanar,E2 reactions occur most rapidly, most frequently with respect to each other at 180∘. This is known as a conformation that is anti-planar. The staggered, anti-planar alignment is favoured as the two σ bonds that become the π bond are aligned.
When nucleofuge and hydrogen are on neighboring carbons in an anti-planar relationship, E2 reactions are preferred. When both Br are in an axial position, it is possible in the specified trans compound. With axial H of C6, Br of C1 can give elimination, and with axial H−C3, Br−C2 can give elimination. The result is 3-bromocyclohexane in both cases, which can then undergo another removal of E2 to give cyclohex 1,3-diene.
Note:
With the symbol Br and the atomic number thirty five, bromine is a chemical element. It is the third-lightest halogen and, at room temperature, is a smoky red-brown liquid that quickly evaporates to form a similarly coloured gas. Therefore, its properties are intermediate between those of iodine and chlorine.
A type of reaction mechanism which is common in organic chemistry is the SN2 reaction. One bond is broken in this process and one bond is synchronously formed, i.e., in one step. SN2 is a sort of reaction mechanism for nucleophilic substitution, the name referring to the mechanism's Hughes-Ingold symbol.
